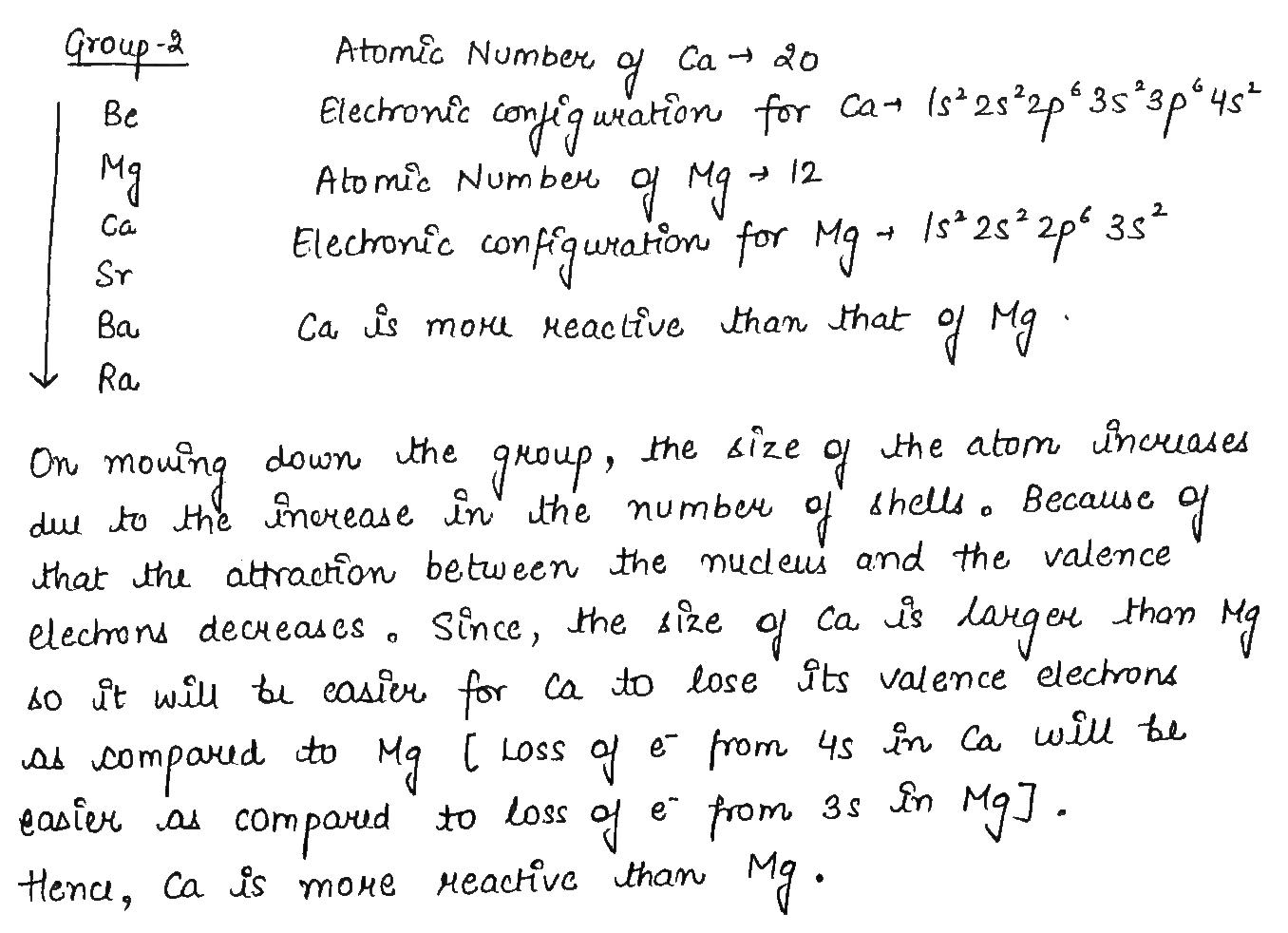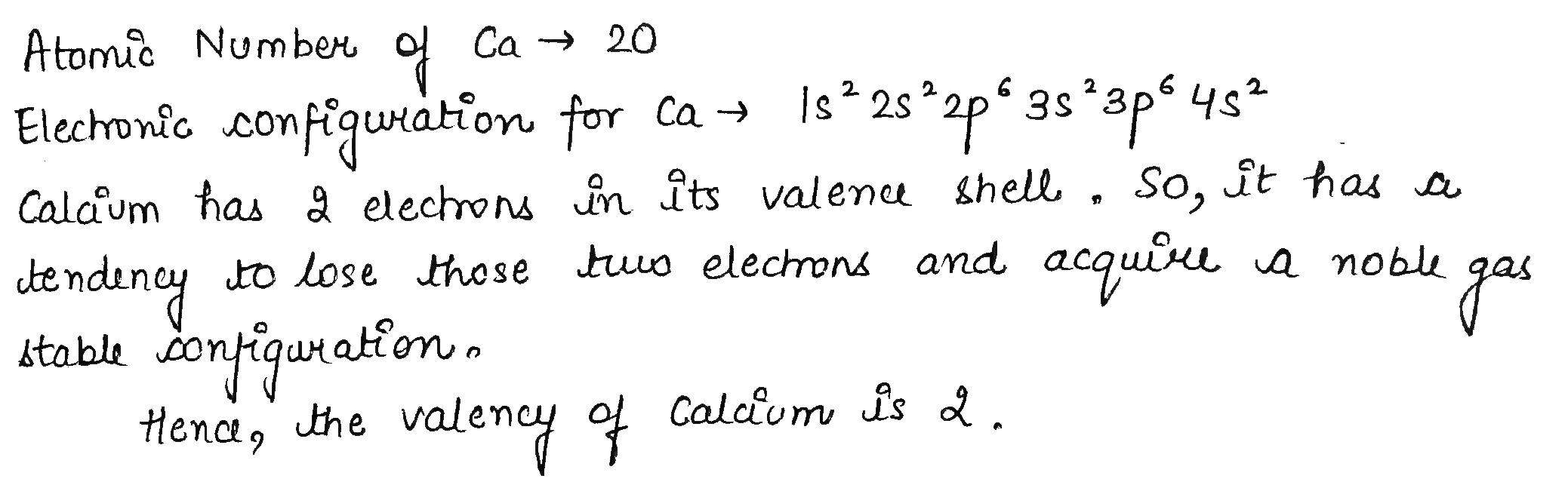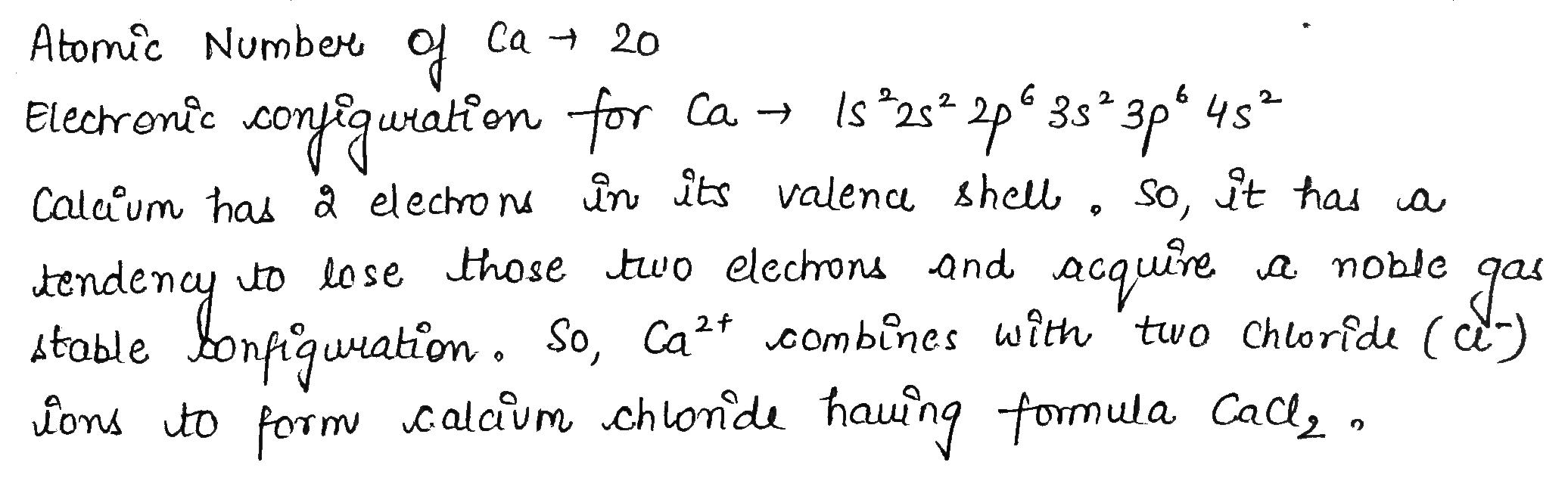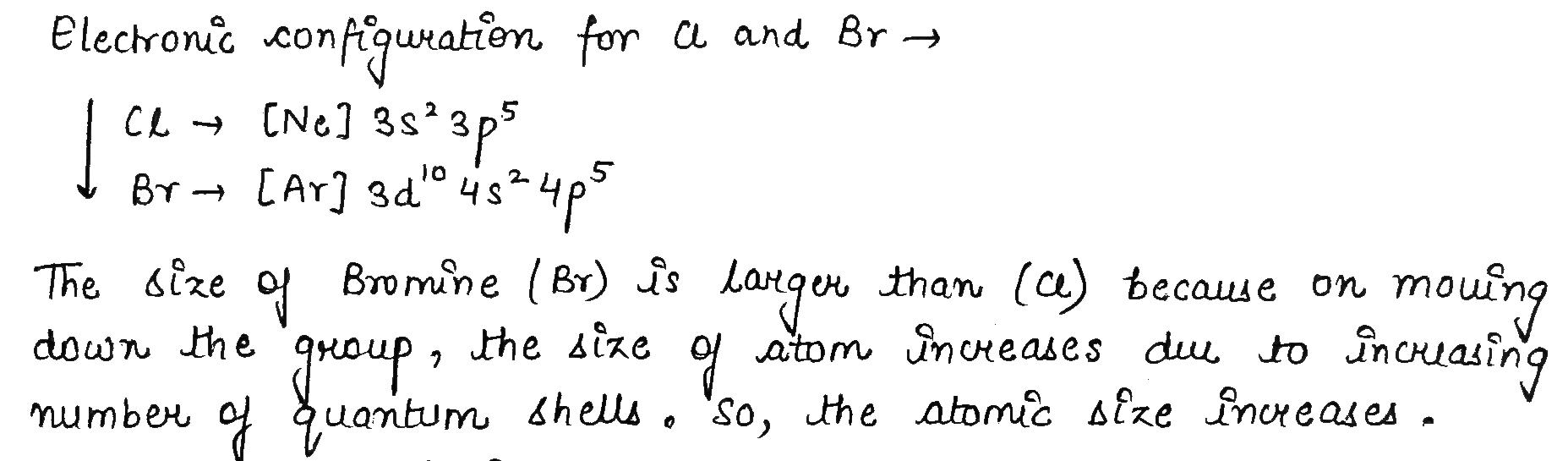The S-Block Elements - Class 11 Medical Chemistry - Extra Questions
"Na+ (cation) is smaller than Na (atom)."
Answer whether the above statement is true or false.
If true enter 1, else enter 0.
Out of Be2+, Mg+ and Ca, Be2+ has maximum IE.
If true enter 1, else enter 0.
The single-bonded metallic radius of sodium is 157 pm. Assume that the increment between radii of different magnitudes is 60 pm.The van der Waals' radius Na in pm is (209+x). Find value of x.
Hydration energy of Mg2+ is more than Li+.
If true enter 1, else enter 0.
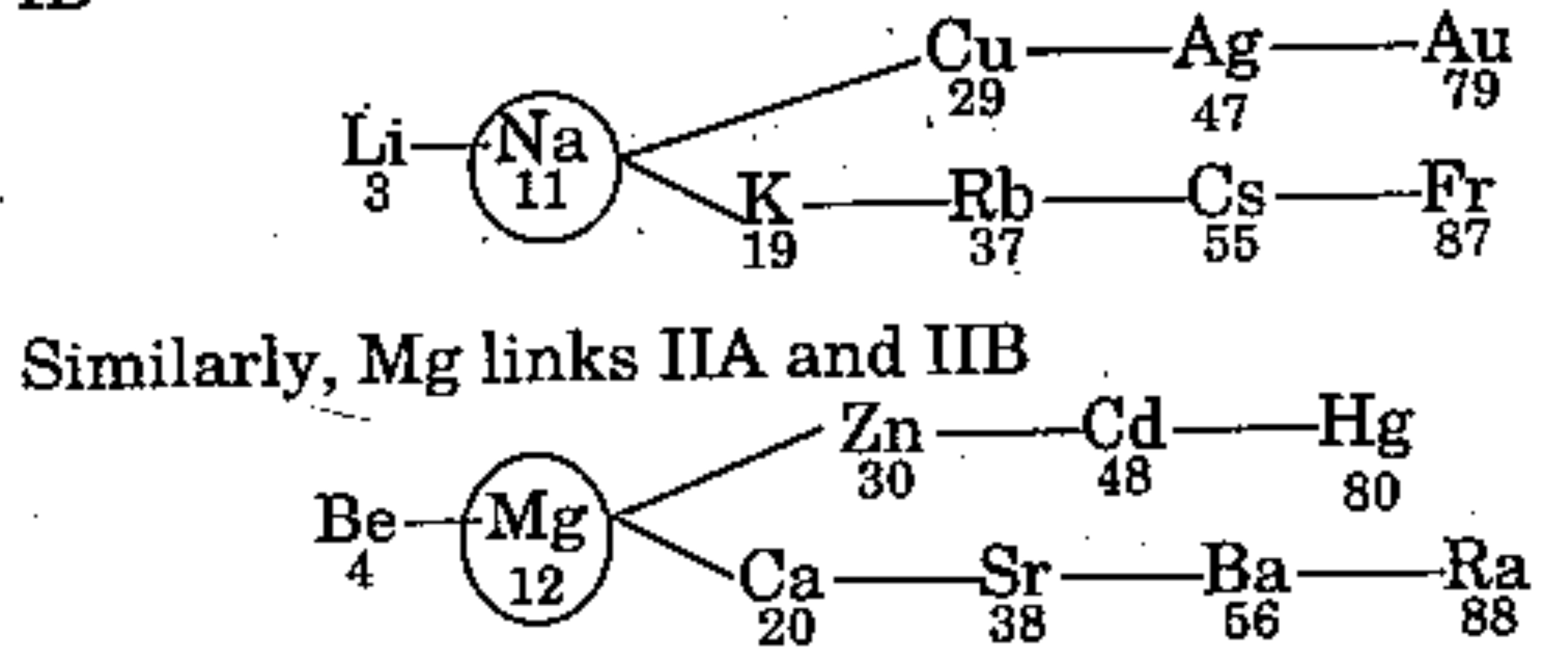
"Na and Mg are also called bridge element since it links Group IA and Group IB".
State whether the above statement is true or false.
Compare Oxidation state of Li and Be.
Chlorophyll a biologically important molecule in which magnesium atoms are held in a position by organic groups.
If true enter 1, else enter 0.
On heating carbonates of Li and Mg, what product will be formed?
(a) Describe how the following properties vary on descending group IA and IIA (alkali and alkaline earth metals) and state how these properties differ between the two groups?
(i)(IE)1and(IE)2
(ii) cationic radii
(iii) rates of reaction with cold water
(b) For the same s-block elements, discuss:
(i) the action of heat on their nitrates
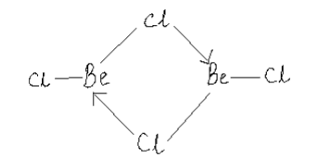
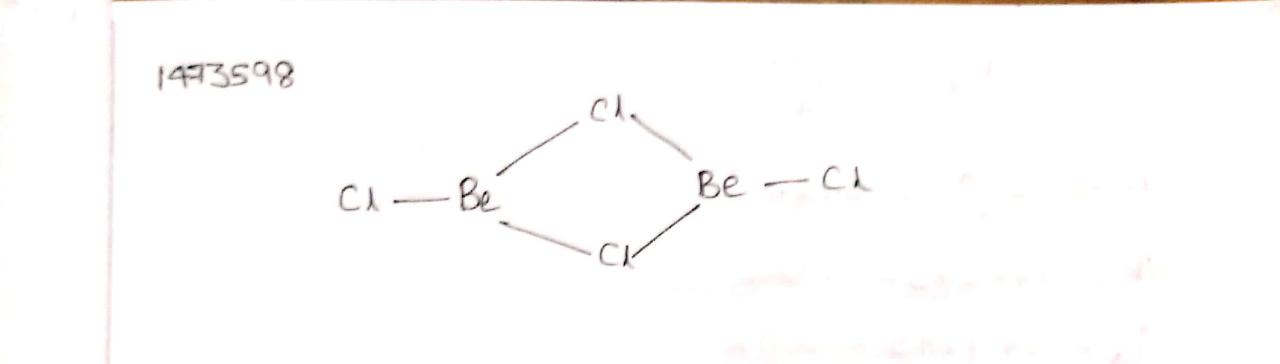
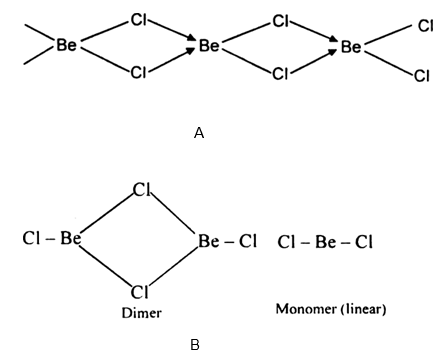
(ii) the crystal structures of their chlorides, XY type only.
Consider the following statement:
Both Li and Mg forms hydrated chloride.
If true enter 1, else enter 0.
State whether the given statement is true or false:
"The polarizing power of Li+ is greater than Na+."
Explain, why electronegativity of Li is greater than Mg?
"The tendency of Li and Mg to form nitrides is such that Li forms nitride whereas Mg does not."
Answer whether the above statement is true or false.
If true enter 1, else enter 0.
"LiCl is covalent while NaCl is ionic."
Answer whether the above statement is true or false.
If true enter 1, else enter 0.
"Both Li and Mg forms Basic oxides."
Answer whether the above statement is true or false.
If true enter 1, else enter 0.
Match the classification (in List I) with the element (in List II).
"Li metal is used in photoelectric cells, while Cs is not used."
Answer whether the above statement is true or false.
If true enter 1, else enter 0.
"Lithium is the only metal among alkali metals that can form nitride."
Answer whether the above statement is true or false.
If true enter 1, else enter 0.
"Polarising power of Li+ ion is nearly the same as that of Mg2+ ion."
Answer whether the above statement is true or false.
If true enter 1, else enter 0.
"When aqueous KO2 solution reacts with CO2, O2 is formed and thus, KO2 is used in submarines."
Answer whether the above statement is true or false.
If true enter 1, else enter 0.
"Carnallite is KCl⋅MgCl2⋅6H2O."
If true enter 1,if false enter 0
"Li of group IA and Be of group IIA show diagonal relationship."
Answer whether the above statement is true or false.
If true enter 1, else enter 0.
"The lattice energies of alkaline earth metal salts are much higher than these of alkali metal salts."
Answer whether the above statement is true or false.
If true enter 1, else enter 0.
The alkali metals follow the noble gases in their atomic structure. What properties of these metals can be predicted from this information?
"Be and Al are made passive by HNO3."
Answer whether the above statement is true or false.
If true enter 1, else enter 0.
"Li of group IA and Mg of group IIA show diagonal relationship."
Answer whether the above statement is true or false.
If true enter 1, else enter 0.
"Of the alkali metals, only Na forms nitrides."
Answer whether the above statement is true or false.
If true enter 1, else enter 0.
"The enthalpy of hydration decreases in going from Li+ to Cs+."
Answer whether the above statement is true or false.
If true enter 1, else enter 0.
"KO2 is O2 absorber and CO2 producer." It is also used in submarines."
Answer whether the above statement is true or false.
If true enter 1, else enter 0.
Compare the solubility and thermal stability of alkali metals carbonates with those of the alkaline earth metals nitrates.
"Be and Al doesnot show diagonal relationship."
Answer whether the above statement is true or false.
If true enter 1, else enter 0.
"Li3N is decomposed by water forming NH3 gas."
Answer whether the above statement is true or false.
If true enter 1, else enter 0.
"When sodium metal is exposed to atmosphere, it reacts with air and forms sodium hydroxide film which absorbs CO2 from air and forms sodium bicarbonate."
Answer whether the above statement is true or false.
If true enter 1, else enter 0.
Na+ is coloured.
If the given statement is correct enter 1 else enter 0.
"Ca3(PO4)2 is the primary constituent of teeth and bones."
Answer whether the above statement is true or false.
If true enter 1, else enter 0.
"The chief factor responsible for the anomalous behavior of lithium is its small size."
Answer whether the above statement is true or false.
If true enter 1, else enter 0.
"Be is not readily attacked by acids due to formation of oxide layer."
Answer whether the above statement is true or false.
If true enter 1, else enter 0.
"The thermal stability of group II oxysalts is more than those of the group I oxysalts."
Answer whether the above statement is true or false.
If true enter 1, else enter 0.
Lithium is the only alkali metal to form a nitride directly.
Write the chemical equation.
Write the chemical equation.
"Potash magnesia is K2SO4.MgSO4.6H2O."
Answer whether the above statement is true or false.
If true enter 1, else enter 0.
Compare the chemistry of group 1 metals with that of group 2 metals with respect to the following:Solubility of carbonates improves on going from group 1 to group 2.
Compare and contrast the chemistry of group 1 metals with that of group 2 metals with respect to the following:
Polarizing power of cations increases on going from group 1 to group 2.
Sodium is found more useful than potassium. If true enter 1,if false enter 0.
Compare and contrast the chemistry of group 1 metals with that of group 2 metals with respect to oxides.
Explain how does the melting point change on moving from group-1 to group-2 in the periodic table?
Why are sodium and potassium kept in kerosene oil ?
What is the most common oxidation state of alkali metals?
Match the column.
Statement: Barium sulphate is sparingly soluble in water whereas beryllium sulphate is freely soluble.
Enter 1 if the given statement is true if false enter 0
Write the word equation and the chemical equation for Magnesium ribbon burned in air.
Alkaline earth metals have higher melting points than alkali metals.
If this is true enter 1, if false enter 0.
Metallic bonding is much stronger in alkali metals compared to alkaline earth metals.
If this is true enter 1, if false enter 0.
The hydroxides and carbonates of sodium and potassium are easily soluble in water while the corresponding salts of magnesium and calcium are sparingly soluble in water. Explain?
Why are potassium and caesium used, rather than lithium used in photoelectric cells?
Why are lithium salts commonly hydrated and those of the other alkali ions usually anhydrous?
Beryllium and magnesium do not give colour to flame whereas other alkaline earth metals do so. Why?
Explain why can alkali and alkaline earth metals not be obtained by chemical reduction methods?
Beryllium doesnot exhibit covalency beyond 4.
If this is true enter 1, if false enter 0.
Write reactions to justify amphoteric nature of aluminium.
Identify the diagonal neighbours in the periodic table.
Calcium floats over water during its reaction with water. Give reason.
Identify the following substances which are underlined:An alkaline gas which produces dense white fumes when reacted with hydrogen chloride gas.
What is the number of products formed on heating nitrates of lithium and magnesium?
Write any two biological importance of Magnesium.
Mention any three diagonal relationship similarities between Berylium and Aluminium.
Comment on the existance of Be2.
Thus , lithium shows similarities to magnesium and beryllium to aluminium in many of their properties. This type of diagonal similarity is commonly referred to as diagonal relationship in the periodic table. The diagonal relationship is due to the similarity in ionic sizes and / or charge / radius ratio of the elements.
BeO is almost insoluble but BeSO4 is soluble in water. Explain.
Lithium differs from other elements of the group. Why?
Name the three metals which react with cold water to form hydrogen gas. Support your answer with equally balanced chemical equations.
Write any four differences between Lithium and other alkali metals.
Li on heating with NH3 forms Li3N whereas Na does not form Na3N. Explain.
(a)Licl is hydrated, but NaCl is always anhydrous. Why ?
(b) Why is it that although Li+ is far smaller than the othe metals ions, it moves through a solution less rapidly than the others ?
(a) BeCl2 in aqueous solution exists as [Be(H2O)4]2+ rather than Be2+ . This is acidic in nature. Explain
(b) Mg3N2 when reacted with water gives NH3 but HCl is not obtained from MgCl2 on reaction with water at room temperature.
What is the diagonal relationship? Give one example.
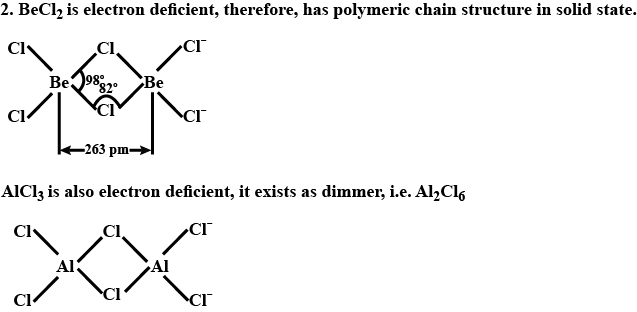
Draw a structure of BeCl2 in vapour phase.
Among KO2,AlO2−,BaO2 and NO+2 , unpaired electron is present in:
Rewrite the following by inserting appropriate word/words
(i) Magnesium Nitride reacts with water to liberate Ammonia
(ii) Lead bromide conducts electricity.
(iii) Starch iodide paper turns blue black in the presence of chlorine .
(iv) Hydrogen chloride molecule contains a covalent bond.
(v) Acid salts are formed by replacement of the ionisable hydrogen ions of the acid by a metallic ion or ammonium ion.
Calcium is an element with the atomic number 20.
(i) Will it be a metal or non-metal?
(ii) What will be its valency?
(iii) What would be the formula of its chloride?
(iv) Will it be smaller or larger than potassium?
(i) Will it be a metal or non-metal?
(ii) What will be its valency?
(iii) What would be the formula of its chloride?
(iv) Will it be smaller or larger than potassium?
Why should a magnesium ribbon be cleaned before it is burnt in air?
Draw the structure of BeCl2 in vapor phase.
I heard that magnesium is found in plants. In what form is it found in them ?
Nitrogen, N2, is the most abundant gas in the Earth's atmosphere and is very unreactive.
Magnesium and lithium both form nitrides with N2. These compounds both contain the N3− ion.
(i) Write an equation for the reaction of magnesium with N2 to form magnesium nitride.
(ii) Solid lithium nitride, Li3N, reacts with water according to the following equation.
Li3N(s)+3H2O(I)→3LiOH(aq)+NH3(aq)
State one observation you would make during this reaction.
Why are group I elements :
(a) univalent
(b) largely ionic
(c) strong reducing agents
(d) poor complexing agents?
(e) why do they have the lowest first ionization energy values in their periods?
Lithium is the smallest ion in group I. It would, therefore, be expected to have the highest ionic mobility, and hence the solution of its salts would be expected to have a higher conductivity than solutions of caesium salts. Explain why this is not so?
Why are the group I metals soft, low melting and of low density ?
Explain the difference in reactivity of the group I metals with water.
All compounds of alkali metals are easily soluble in water but lithium compounds are more soluble in organic solvents. Explain.
Why are BeSO4 and MgSO4 readily soluble in water while SrSO4and BaSO4 are insoluble?
Describe how you would make lithium hydride. Give equation to show two important properties of lithium hydride. The compound contains the isoelectronic ions Li+ and H− which ion is the larger and why?
Why and in what ways does lithium resemble magnesium ?
Lithium resembles magnesium in some of its properties. Mention two such properties and give reasons for this resemblance.
Identify the final product in each reaction:
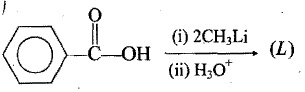
What is the reason for lithium having a greater tendency to form covalent compound than the other elements in the group ?
Write the balanced chemical equations for the following reaction: Sodium iodate is added to a solution of sodium bisulphite.
Give reasons for the following in one or two sentences only: "BeCl2 can be easily hydrolyzed".
Why do beryllium and magnesium not impart colour to the flame in the flame test?
Name the alkaline earth metal which shows resemblance with aluminium.
The s− block elements are characterized by their larger atomic sizes, lower ionization enthalpies, invariable + 1 oxidation state and solubilities of their oxosalts. In the light of these features describe the nature of their oxides, halides, and oxosalts.
Arrange the following in increasing order of the property indicated:
Li, Na, K, Rb and Cs (hydrated radii)
Answer the following:
Which element of alkaline earth metal has least density?
The crystalline salts of alkaline earth metals contain more water of crystallization than the corresponding alkali metal salts. Why?
What is the structure of BeCl2 molecule in gaseous and solid state?
Which elements of group 2 do not give characteristic flame colouration?
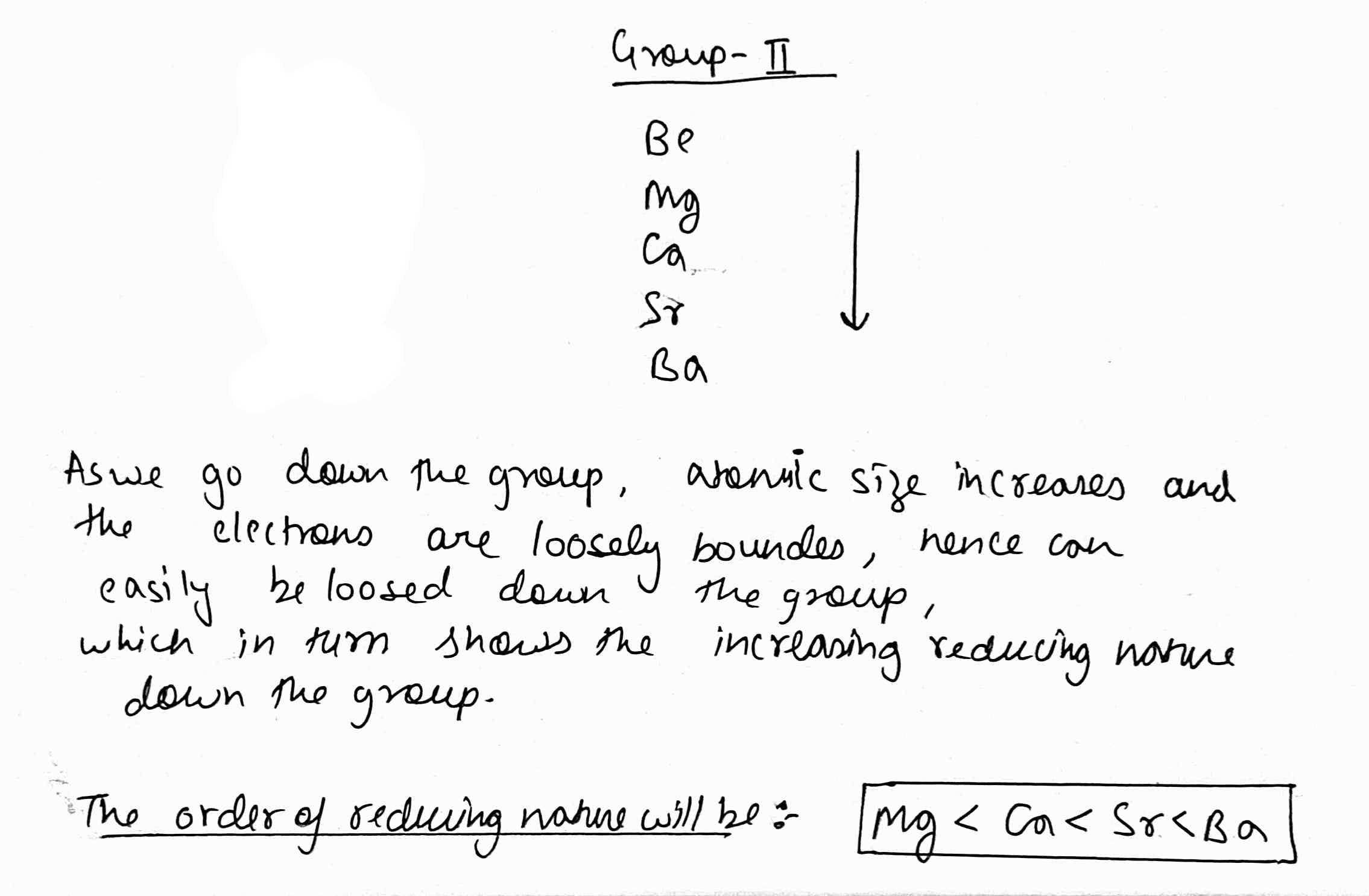
How does reducing nature of the alkaline earth metals change when we move from Be to Ba?
Answer with giving reason:
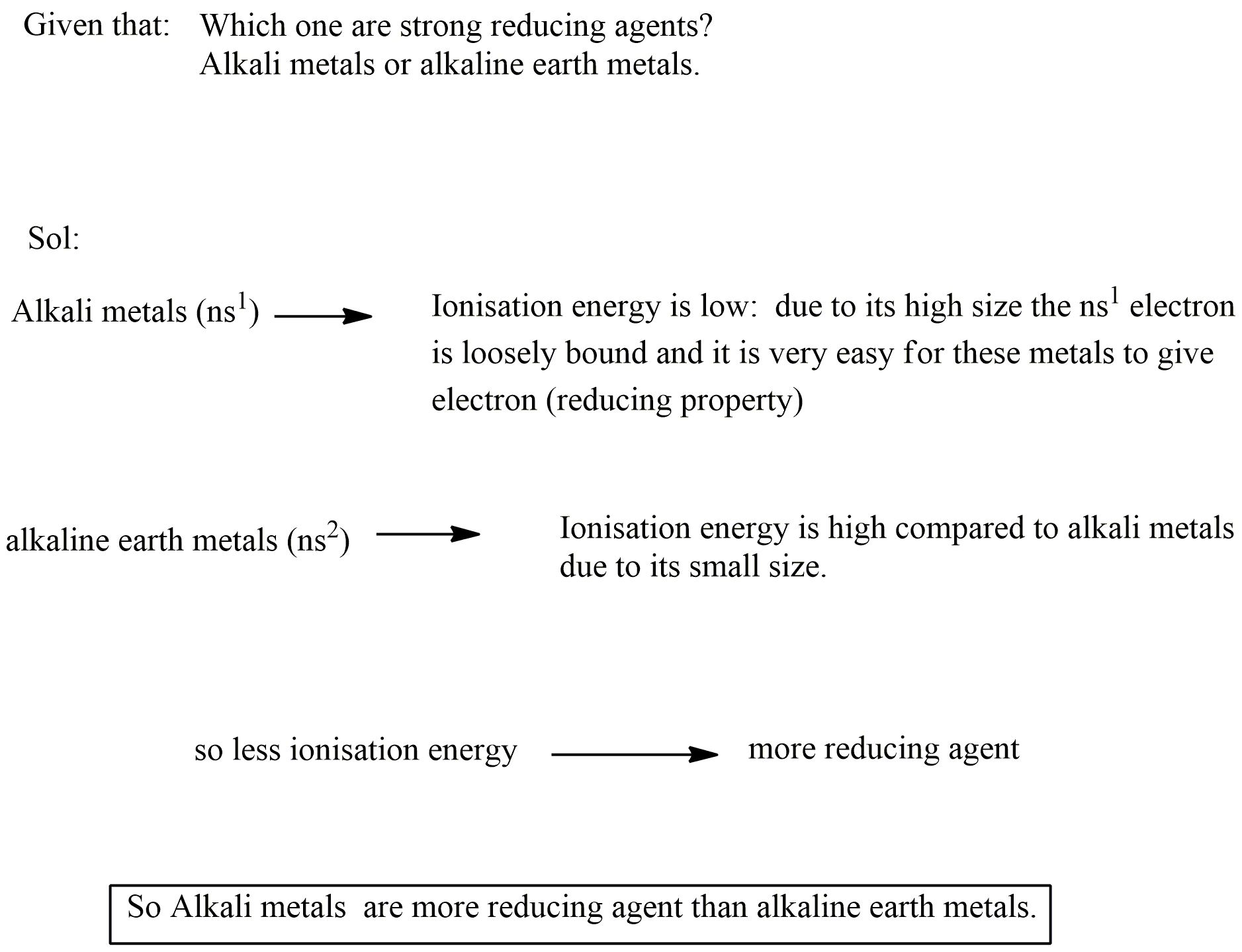
Which one are strong reducing agents?
Alkali metals or alkaline earth metals.
Answer with giving reason:
Which are more electropositive alkali metals or alkaline earth metals?
How do the following properties of the alkaline earth metals change when we move from Be to Ba?
Size of the atoms and ions.
How do the following property of the alkaline earth metals change when we move from Be to Ba?
Ionization energy
Arrange the following as decreasing order of covalent character
LiCl,LiBr and LiI
Answer the following:
Which out of sodium of potassium has higher melting point?
What happens when following are heated?
Lithium carbonate.
Which one is soluble in water, LiF or KF?
Which alkali metal is radioactive in nature?
Answer the following:
Name the alkali metal which shows diagonal relationship?
Answer the following:
Why does table salt get wet in rainy season?
What happens when?
Sodium is strongly heated in oxygen and the product is treated with H2SO4.
What happens when following are heated?
Lithium nitrate.
Match the elements in List-I with their nature in List II
Explain the following with relevant reason.
First ionisation potential of Al is lower than that of Mg.
(a) What is the number of valence electrons in the atoms of first element in a period?
(b) What is the usual number of valence electrons in the atoms of the last elements in a period?
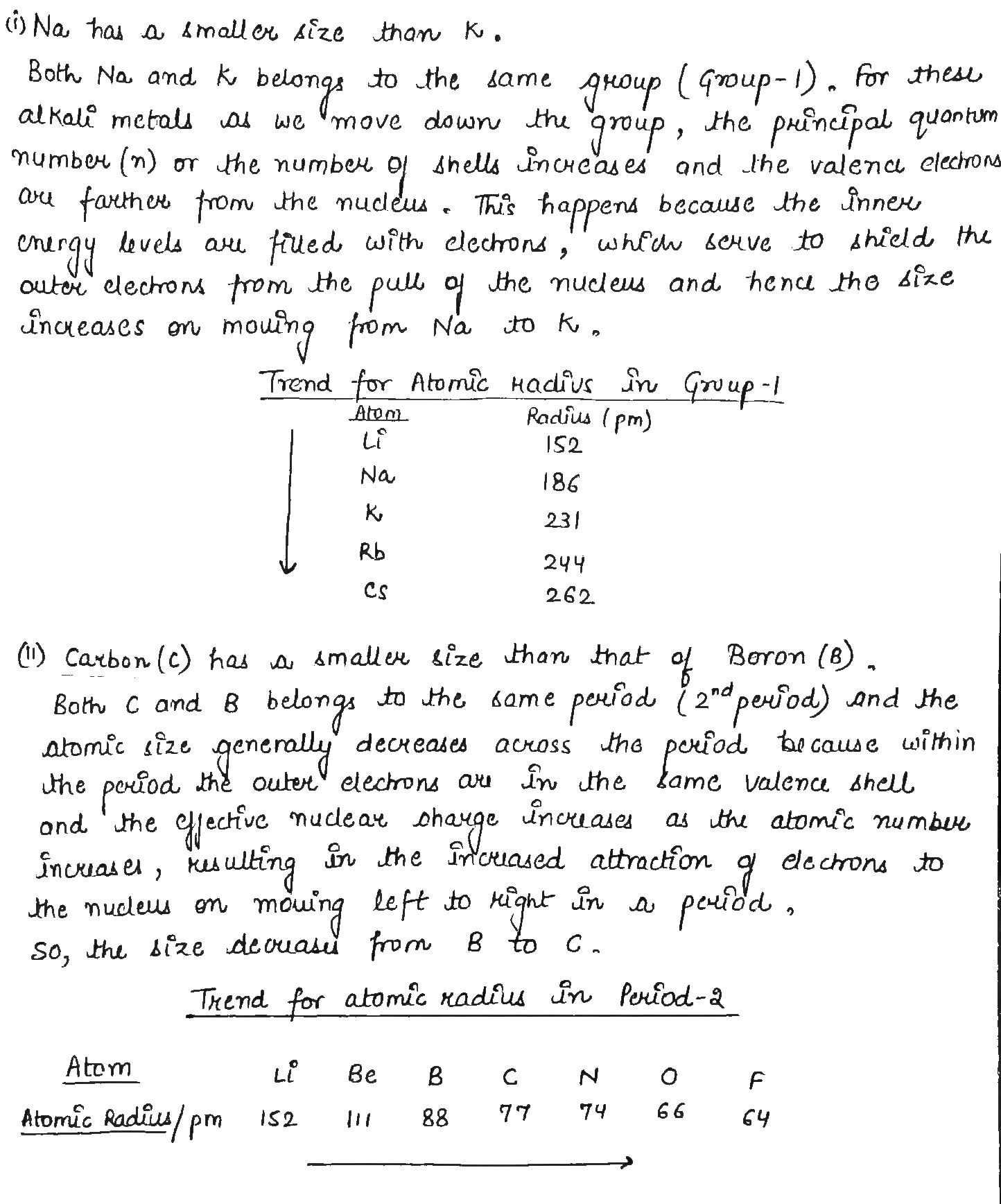
Which has smaller size Na or Na+?
Calcium is an element with atomic number 20.
Is it a metal or non-metal?
Calcium is an element with atomic number 20.
Is it more or less reactive than Mg?
Which one has the bigger size?(i) Na or Cl(ii) Cl or F
Calcium is an element with atomic number 20.
What will be its valency?
Calcium is an element with atomic number 20.
What will be the formula of its Chloride?
Calcium is an element with atomic number 20.
Will it be larger than K or smaller?
Which one has the smaller size?
K(19) or Na(11);B(5) or C(6)
Which has bigger size; Cl or Cl−?
Which one has larger atomic size Cl or Br ? Why ?
Why is the size of sodium greater than magnesium ?
Explain the following :
Size of an atom progressively becomes smaller when we move from sodium (Na) to chlorine (Cl) in the third period of the periodic table.
Match the elements given in Column I with the properties mentioned in Column II.
Why are alkali metals kept in kerosene oil?
An element has 2 electrons in its N shell.
State the name assigned to this group ?
Present a comparative account of the alkali and alkaline earth metals with respect to the following characteristics:
(i) Tendency to form ionic/covalent compounds
Try to find it
...... glass dissolves in water.
The alkali metals have no tendency to show variable oxidation states. Give reason.
Why are group 1 elements called alkali metals ?
Why do alkali metals have low ionisation energy ?
Variation of first ionization enthalpies with Z=1 to 60.
Study the figure given above and answer the following:
(a) Compounds of Xe are known but other noble gases do not form compounds
(b) Giving reason arrange alkali metals in increasing order of first ionization enthalpies
(c) Estimate the first ionization enthalpy values of Mg and Al.

What are alkalies?
Name :
The element of period 3 valency 4.
LiCI and MgCk dissolve in alcohol. How do you explain this?
This question refers to the elements of the periodic table with atomic number from 3 to 18. Some of the elements are shown by letters , but the letters are not the usual symbols of the elements.
| 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| A | B | C | D | E | F | G | H |
| 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| I | J | K | L | M | N | O | P |
- Which of these is an alkali metal ?
Arrange the following in order of increasing radii :
Mg2+,Mg,Mg+
Why does ionisation energy of alkali metals decrease with the increase in atomic number ?
Why are alkali metals used in photoelectric cells ?
Why do alkali metals have low melting and boiling points ?
Why is second ionisation energy of alkali metals higher than alkaline earth metals ?
Why is potassium more reactive than sodium ?
Why do alkali metals not occur in free state ?
Why do alkali metals give characteristic flame colouration?
Write electronic configuration of Na (11) and K (19).
Complete the reaction : Lil+KF→
Why group 2 elements (Mg and Ca) are harder and denser than group 1 elements ?
Discuss the diagonal relationship of Be and Al with regard to
⋅ the action of alkali and
⋅ the structure of the chlorides.
Why does Be resemble Al?
Name the metal which floats on water without apparent reaction.
Why does lithium show anomalous behaviour ?
Give reason for diagonal relationship of lithium with magnesium.
Why is oxidation state of Na and K always + 1?
Name the elements (alkali metals) which form superoxide when heated in excess of air.
Compare four properties of alkali metals and alkaline earth metals.
Give two important ores each of Na and K.
Write balanced equations for the following reactions and name the main product formed in each case,
⋅NaBH4+I2→
⋅B2H6+NaH→
⋅BF3+LiH450K→
⋅SiCl4+H2O→
Discuss the diagonal relationship of Be and Al with regard to
action of alkali and
the structure of their chloride.
Why is it that the s-block elements never occur in free state/nature? What are their usual modes of occurrence and how are they generally prepared?
What are alkali metals? Describe their general properties.
Which out of Li, Na, K, Be, Mg, Ca has lowest ionization enthalpy and why ?
Name an alkali metal carbonate which is thermally unstable and why ? Give its decomposition reaction.
The ionic compounds of alkali metals are colourless, why?
What makes lithium show properties uncommon to the rest of alkali metals ? Write two points of similarly in properties between lithium and magnesium.
Which metal is present in chlrophyll ? How does this metal react with N_2 ?
The E^0 for C1^-/Cl_2 is 1.36, for I^-/I^2 is +0.53, for Ag^+/Ag is + 0.79, Na^+ is -2.71 and for Li^+ / Li is -3.04 V Arrange the following species in decreasing order of reducing strength. I^-, Ag, Cl^- Li, Na
Lithium does not show similarity with other elements of the group. Explain the reasons.
In a period, melting points of alkali metals are lower than alkaline eath metals, why?
What is diagonal relationship? How beryllium shows similarity with Aluminium?
Write balanced equation for relation between
Be_2 C and water
Why potassium and calcium are used in photoelectric all in place of Lithium?
Alkali metals are strong electropositive in nature, Why?
Why lithium compounds are covalent in nature?
Sodium is kept in Kerosene. Why?
Compare the alkali metals and alkaline earth metals with respect to
Ionisation enthalpy
Atomic and ionic radius
Lithium has the highest ionization enthalpy in group I elements, yet it is the strongest reducing agent. why?
Which out of sodium or potassium has higher melting point ?
What is diagonal relationship due to ?
What makes lithium to show properties uncommon to the rest of the alkali metals?
Name the alkaline earth metal which forms covalent compounds.
State any one reason for alkaline earth metals having a greater tendency to form complexes than alkali metals.
Name the alkali metal which shows diagonal relationship with magnesium.
Name the chief factor responsible for the anomalous of lithium.
6 \cdot 70 \mathrm{g} of an alkali metal oxalate was dissolved per litre of the solution. 10 \mathrm{cm}^{3} of this solution required 20 \mathrm{cm}^{3} of 0.01 \mathrm{M} potassium permanganate solution in acidic medium. What is the atomic weight of the alkali metal.
Which alkaline earth metal is radioactive?
Why does beryllium show similarities with aluminium ?
Why alkaline earth metals have a greater tendency to form complexes than the alkali metals?
Fill in the blanks :
lithium resembles more with _____ than with sodium.
Write three general characteristics of the element of s-block of the periodic table which distinguish them from the elements of the other blocks.
Fill in the blanks :
The _______ of Be is highest among the alkali metals.
Fill in the blanks :
Ca^{2+} has a smaller ionic radius than K^+ because it has _______.
Match compounds given in List 1 with their use in List 2
The single-bonded metallic radius of sodium is 157 pm. Assume that the increment between radii of different magnitudes is 60 pm. The covalent radius of Na in pm is (90+x). Find x.
Ca^{2+} can be determined by precipitating it as CaC_2O_4 gravimetrically. The percentage of Ca^{2+} in a sample, if 0.5 g of Ca^{2+} salt gave 1.28g of CaC_2O_4 is
Given (Ca = 40, Ca = 12, O = 16)
The single-bonded metallic radius of sodium is 157 pm. Assume that the increment between radii of different magnitudes is 60 pm. The ionic radius Na^{+} in pm is (90 + x). Find x.
"While comparison of Li^{+} and Na^{+} salts in terms of property of complex formation we found that [Li(NH_3)_4] exists and Na does not form any complex."
Answer whether the above statement is true or false.
If true enter 1, else 0
Entries in List 1 are to be matched with entries of List-Each entry of List-1 may have matching with one or more than one entries in List-2.
Lithium on heating in air forms mainly Li_2O (oxide) and not peroxide (Li_2O_2). If true enter 1 else 0.
Match List I (Compounds) with List II (Associated Uses).
Lithium being heated in air mainly forms the monoxide and not the peroxide. If true enter 1 else 0.
Match the cations from List I with their properties in List II.
Compare the solubility and thermal stability of the following compounds of the alkali metals with those of the alkaline earth metals. (a) Nitrates, (b) Carbonates, (c) Sulphates.
In what ways lithium shows similarities to magnesium in its chemical behaviour?
Explain the significance of sodium, potassium, magnesium and calcium in biological fluids.
Why is LiF almost insoluble in water whereas LiCl is soluble not only in water but also in acetone?
It may be noted that lithium has most negative E^{\Theta} value (table 9.1). But its reaction with water is less vigorous than that of sodium which has the least negative E^{\Theta} value among the alkali metals. This behaviour of lithium is attributed to its small size and very high hydration energy. Other metals of the group react explosively with water.
What is diagonal relationship ? Give two examples to illustrate the concept ?
Write chemical formula of washing soda. How is it obtained from baking soda? Name the one industrial use of washing soda other than washing clothes.
Present a comparative account of the alkali and alkaline earth metals with respect to the following characteristics.a. Tendency to form ionic/covalent compounds
b. Nature of oxides and their solubility in water
c. Formation of oxosalts
d. Thermal stability of oxosalts
How do you account for the strong reducing power of lithium in aqueous solution?
Which of the following methods would you use to extinguish a fire of lithium, sodium or potassium metals? Explain why some of these are unsuitable, and give the reactions involved.
(a) water
(b) nitrogen
(c) carbon dioxide
(d) asbestos blanket
The four general methods of extracting metals are thermal, decomposition, displacement of one element by another, chemical reduction and electrolytic reduction. how are the group I metals obtained and why are the other methods unsuitable?
The stability of peroxide and superoxide of alkali metals increase as we go down the group. Explain giving reason.
Element A burns in nitrogen to give an ionic compound B. Compound B reacts with water to give C and D. A solution of C becomes 'milky' on bubbling carbon dioxide. Identify A, B, C and D.
How metal calcium is manufactured?
Name the element which is invariable bivalent and whose oxide is soluble in excess of NaOH.
Arrange the following:
Sr, Ba, Ca, Mg in order of increasing reducing nature.
How does the oxidation potential of the alkaline earth metals change when we move from Be to Ba?
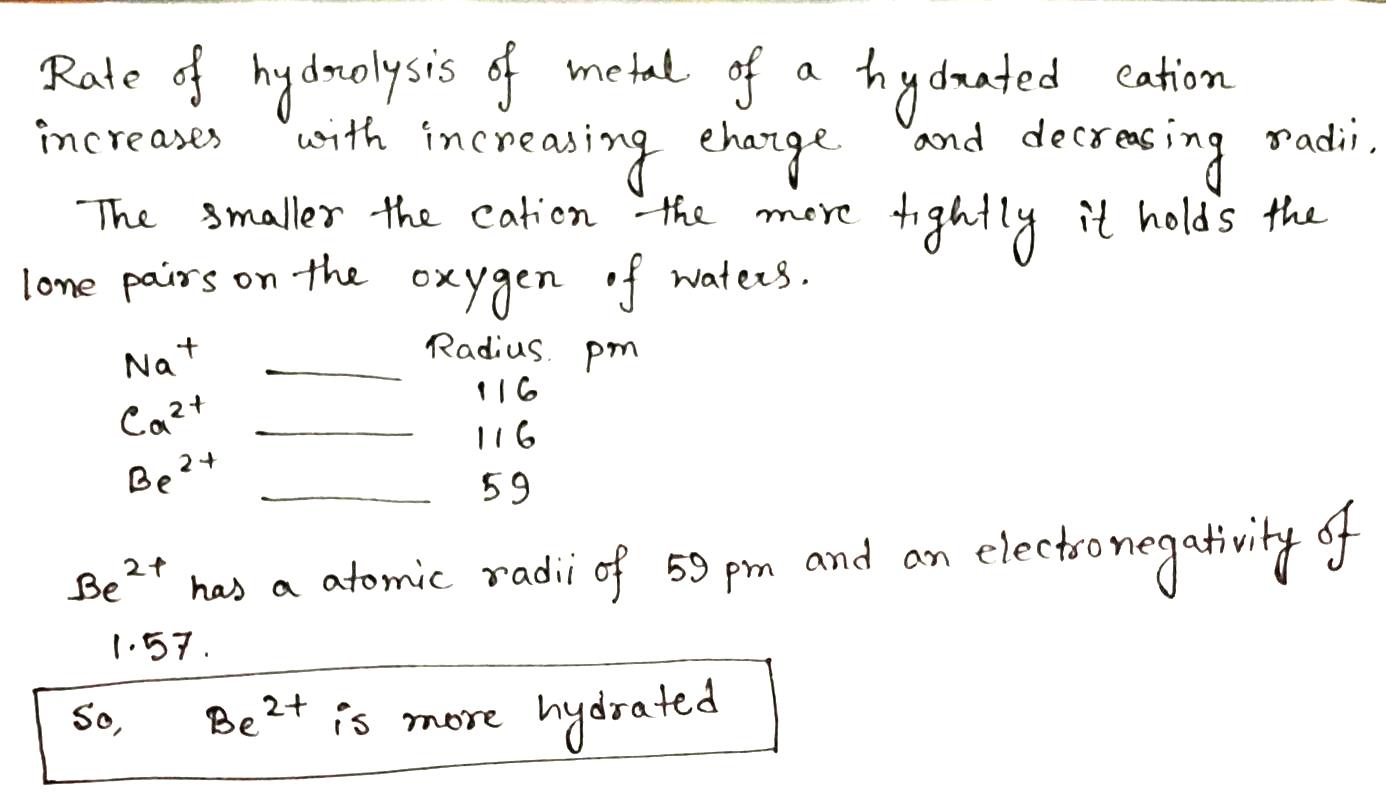
Answer with giving reason:
Which is more hydrated?
Be^{2+}, Na^+, Ca^{2+}
Give reasons:
LiBr has lower melting point than LiF.
Answer the following:
What makes lithium to show properties different than other alkali metals?
Give reasons:
LiCl is soluble in alcohol.
Give reasons:
MgCl_{2} is more than covalent than NaCl
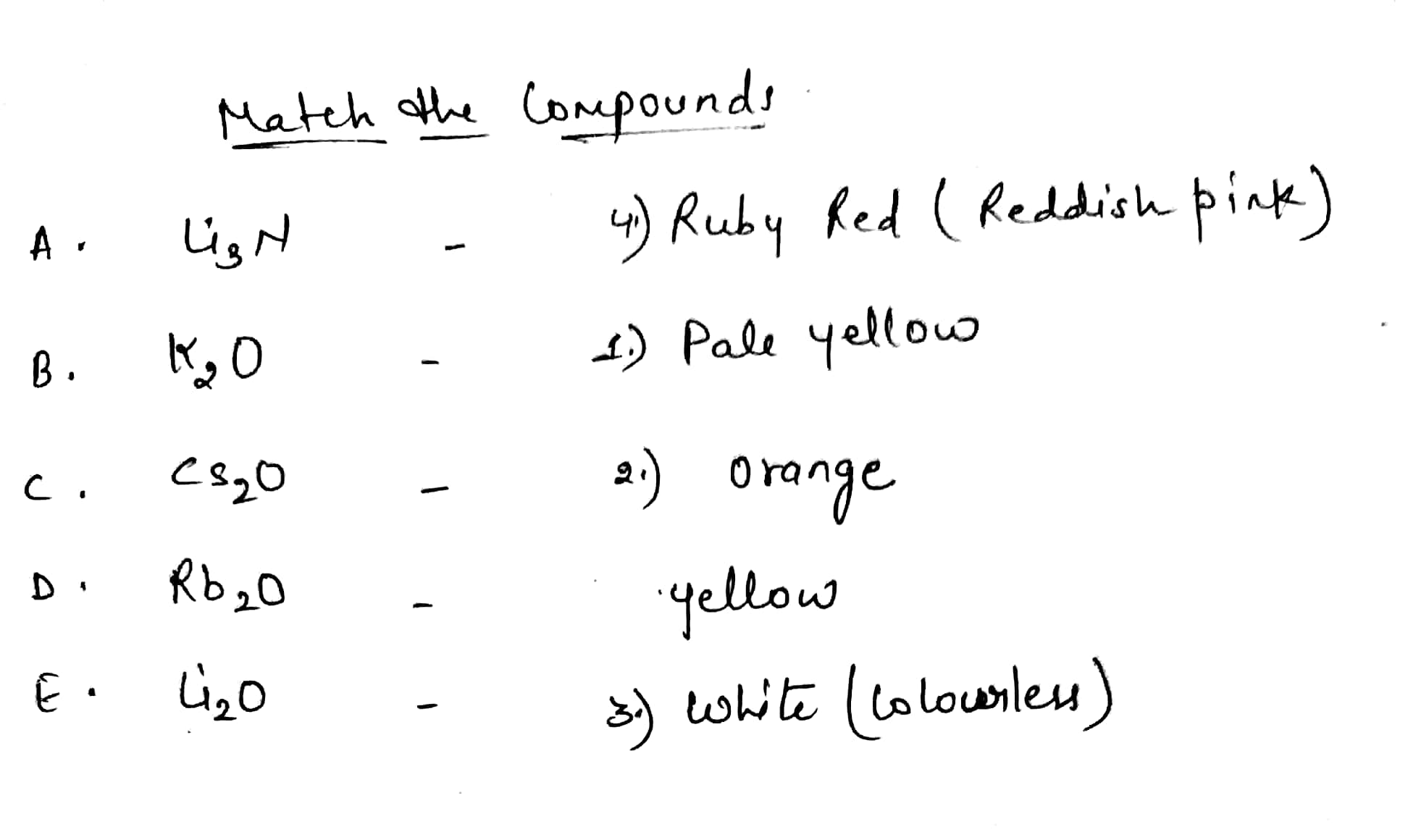
Match the compounds in List-I with their colour in List-II:
Answer the following:
Name the alkali metals which form superoxides when heated in excess of air.
Complete and balance the following equations:
KO_{2}+H_{2}O\rightarrow \ ......\ +\ O_{2}
Match List I with List II
Match the compounds listed in column-I with the related uses/particulars listed in column-II.
Name two elements you would expect to show chemical reactions similar to calcium. What is the basis of your choice ?
Name:(a) A yellow monoxide that dissolves in hot and concentrated caustic alkali.
(b) A white, insoluble oxide that dissolves when fused with caustic soda or caustic potash.
(c) A compound containing zinc in the anion.
Alkali metals have low ionization energy why?
Alkali metals are paramagnetic but their salts are diamagnetic. Explain.
Why alkali metals are normally kept in kerosene oil ?
When is a cation highly polarising ? which alkali metal has the highest polarising power ?
Why is sodium metal kept under kerosene oil ?
Why is calcium preferred over sodium to remove the last traces of moisture from alcohol?
Why is it that on being heated in excess supply of air , K , Rb and Cs from the superoxides in preference to oxides and proxides?
Why cesium can be used in photoelectric cell while lithium cannot be ?
Among alkali metals in aqueous solution Li^+ ion has the lowest mobility. Why?
Among alkali metals , why is lithium regarded as most apt reducing in aqueous solutions?
State as to why
(a) A solution of Na_2CO_3 is alkaline
(b) Alkaline metals are prepared by electrolysis of their fused chlorides
(c)Sodium is found more useful than potassium.
Comment on each of the following observations :
The mobilities of alkali metal ions in aqueous solution are : Li^+ < Na^+ < K^+ < Rb^+ < Cs^+
Like lithium in group I beryllium shows anomalous behaviour in groupWrite three such properties of beryllium which make it anomalous in the group?
Why are alkali earth metals good reducing agents ?
Calgon is the trade name of ..............
Give three important uses of each of calcium and magneisum.
Beryllium exhibits some similarities with aluminium. Point out three such properties.
Give the reason for the following?
Be and Mg do not impart colour to the flame.
Class 11 Medical Chemistry Extra Questions
- Chemical Bonding And Molecular Structure Extra Questions
- Classification Of Elements And Periodicity In Properties Extra Questions
- Environmental Chemistry Extra Questions
- Equilibrium Extra Questions
- Hydrocarbons Extra Questions
- Hydrogen Extra Questions
- Organic Chemistry Some Basic Principles And Techniques Extra Questions
- Redox Reactions Extra Questions
- Some Basic Concepts Of Chemistry Extra Questions
- States Of Matter Gases And Liquids Extra Questions
- Structure Of Atom Extra Questions
- The P-Block Elements Extra Questions
- Thermodynamics Extra Questions
- The S-Block Elements Extra Questions
 Li2O(s) + 2 NO2(g) + O2(g)
Li2O(s) + 2 NO2(g) + O2(g)