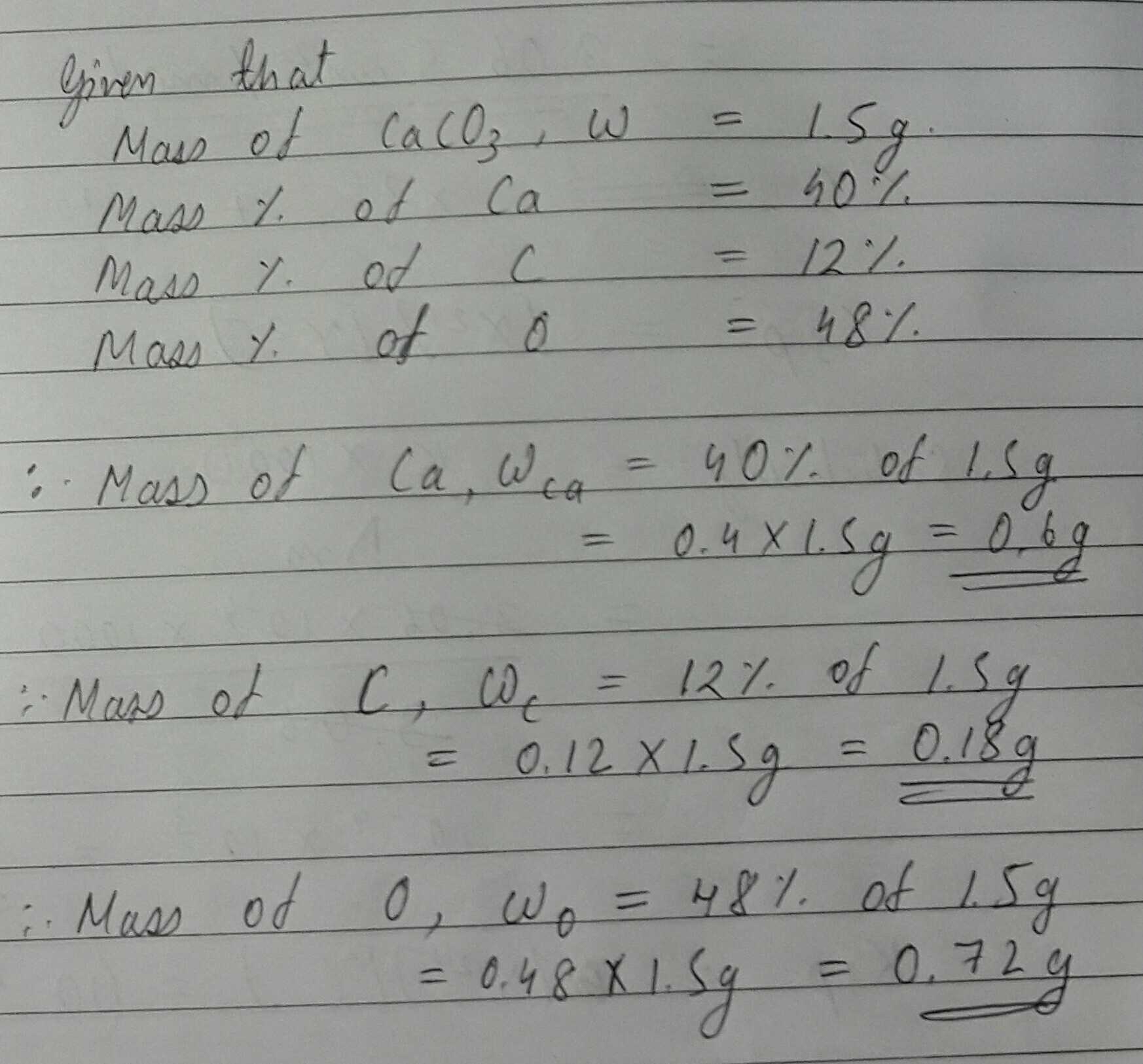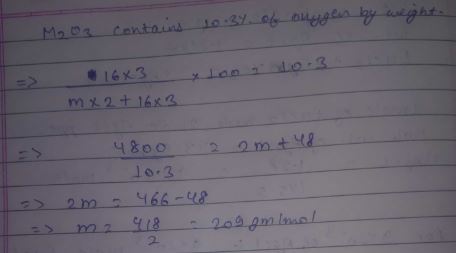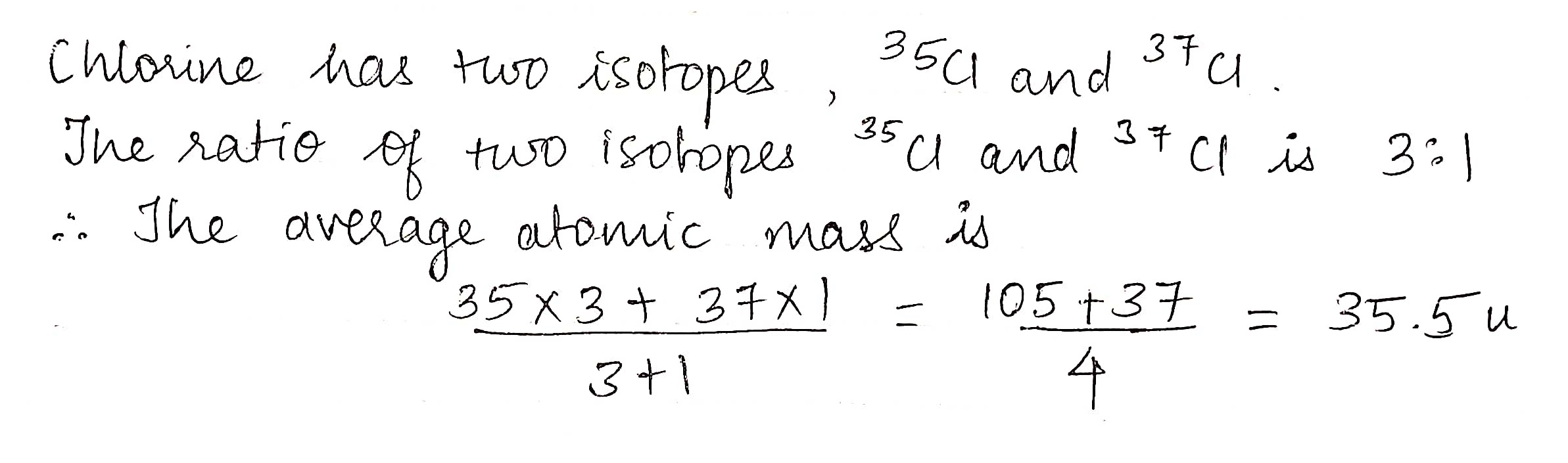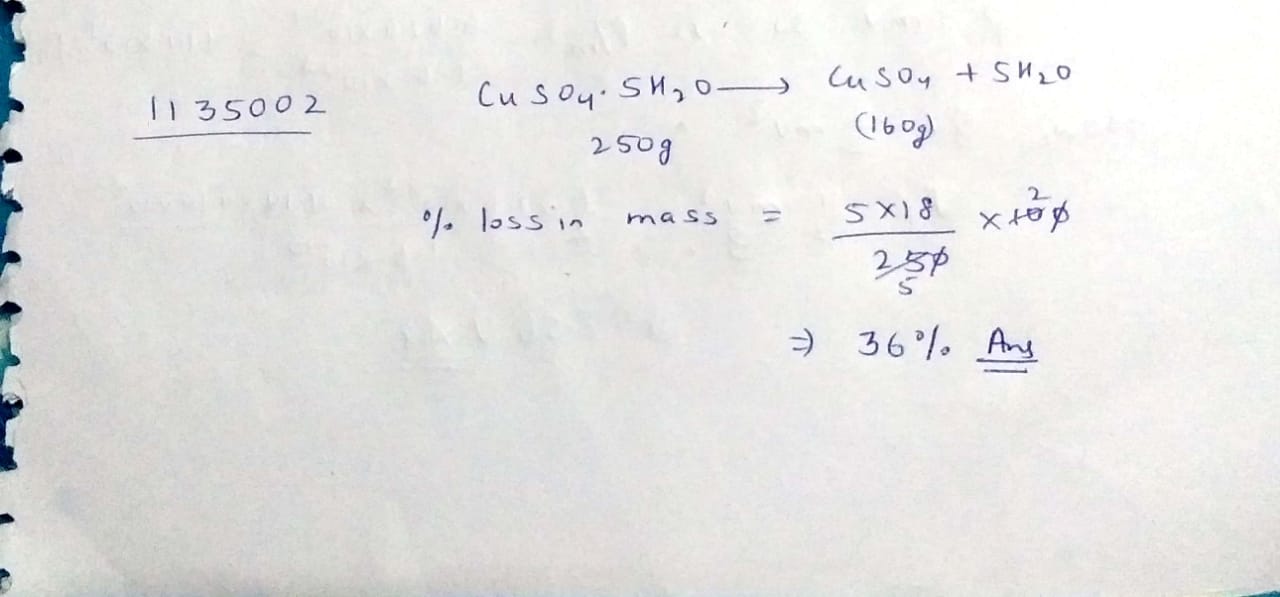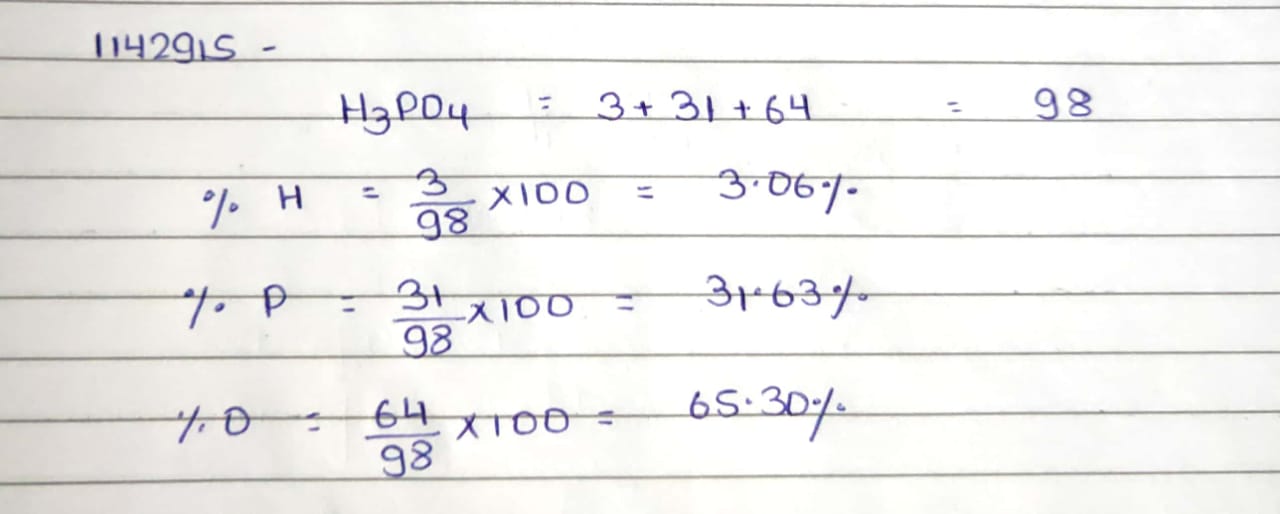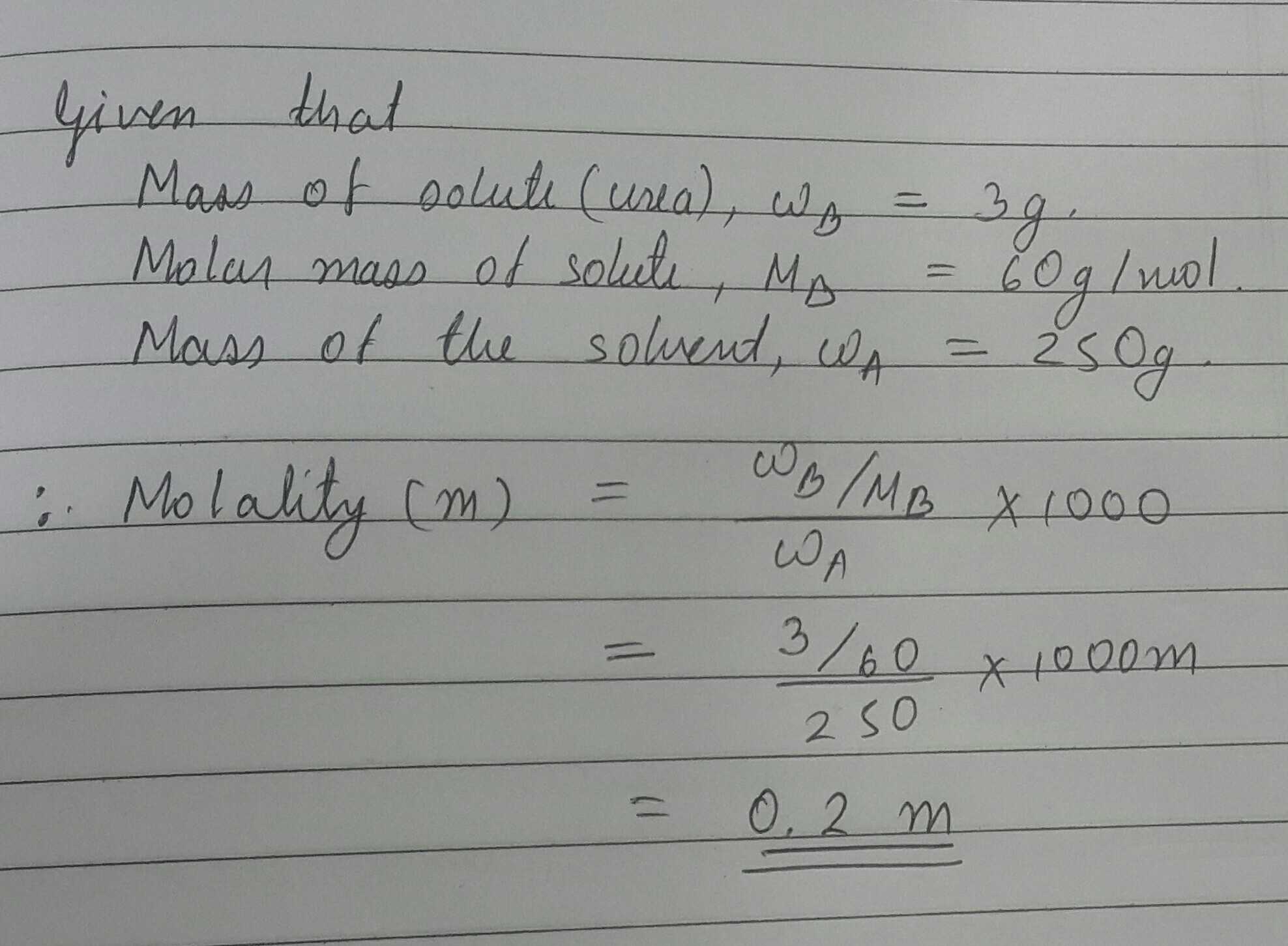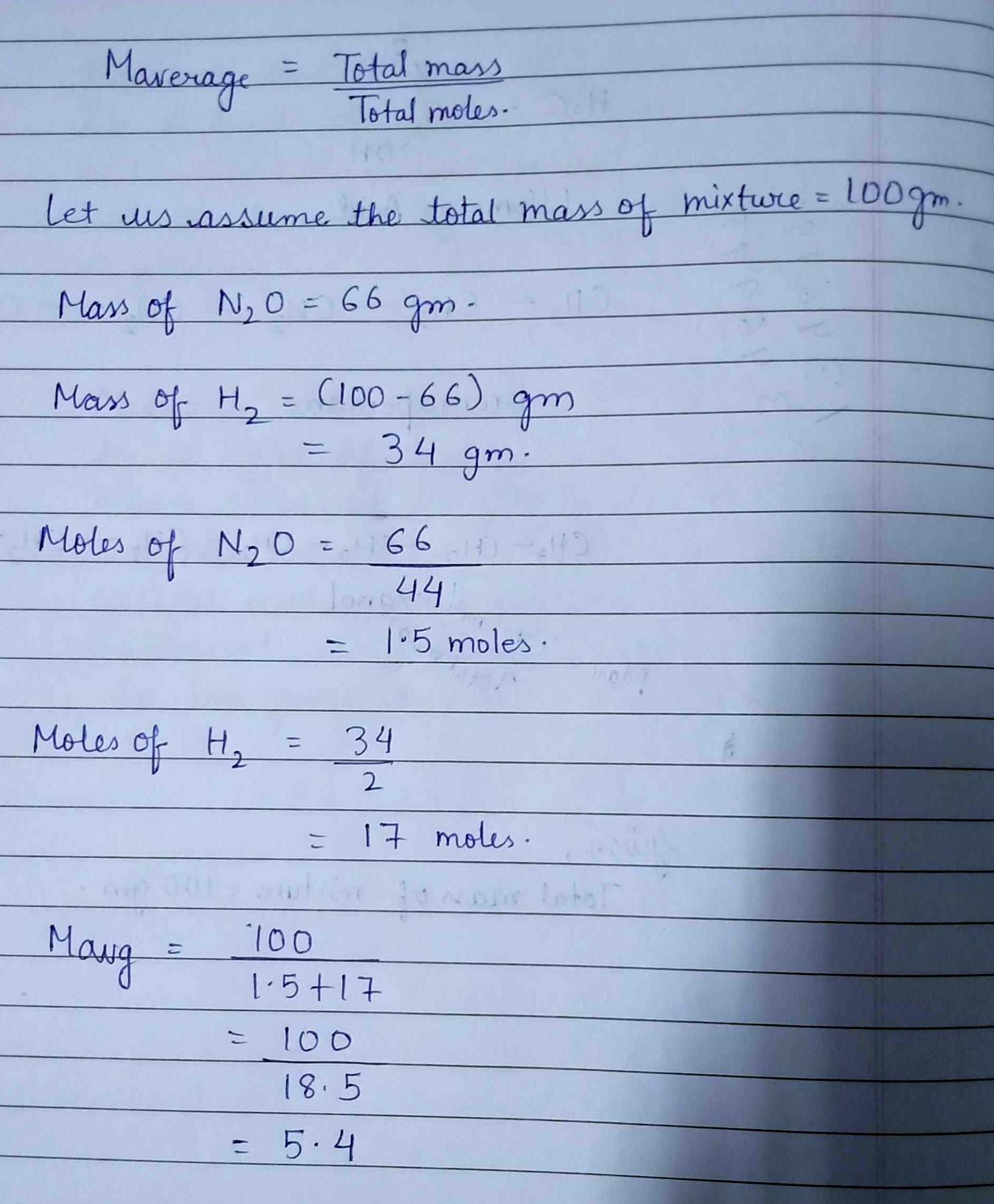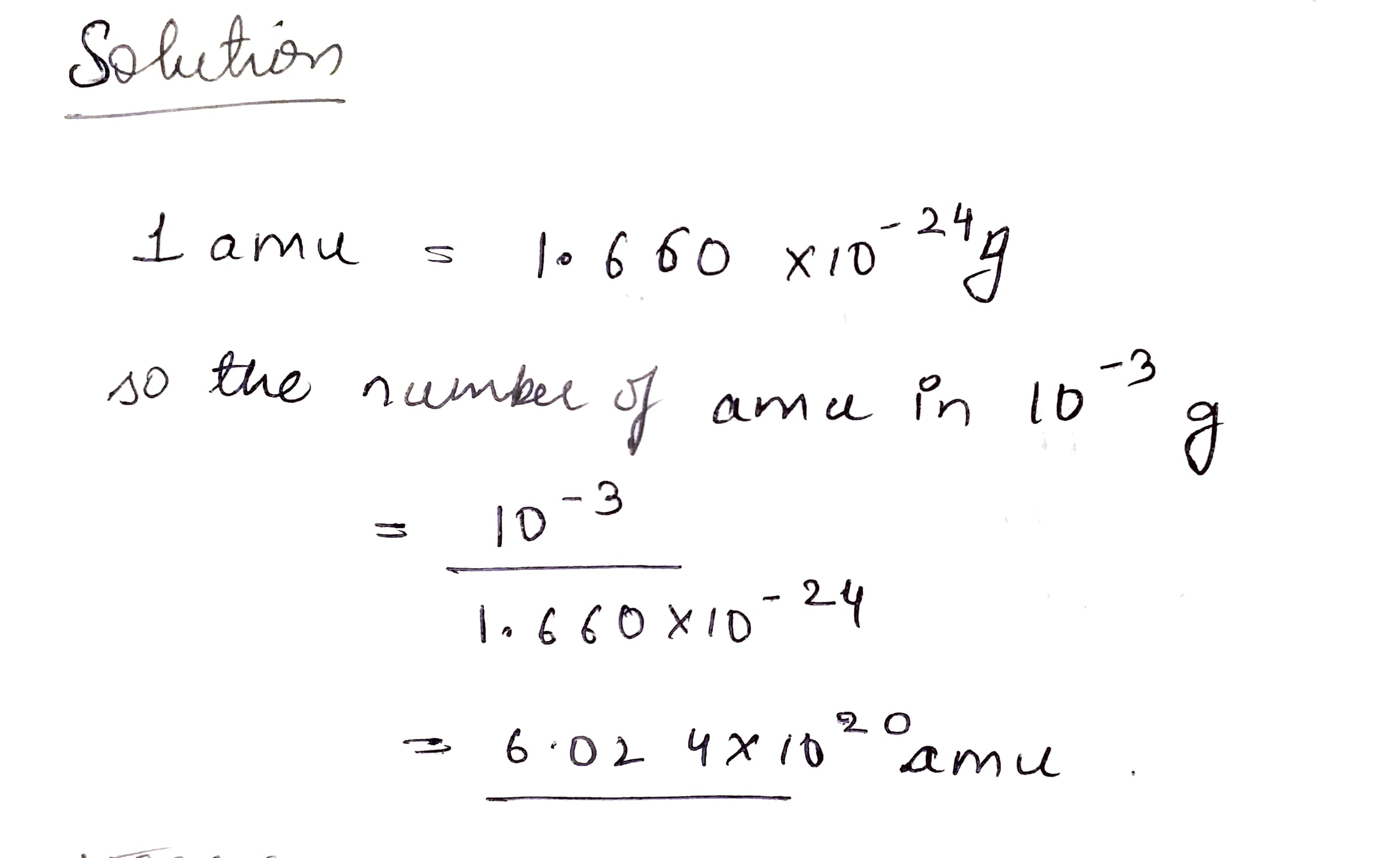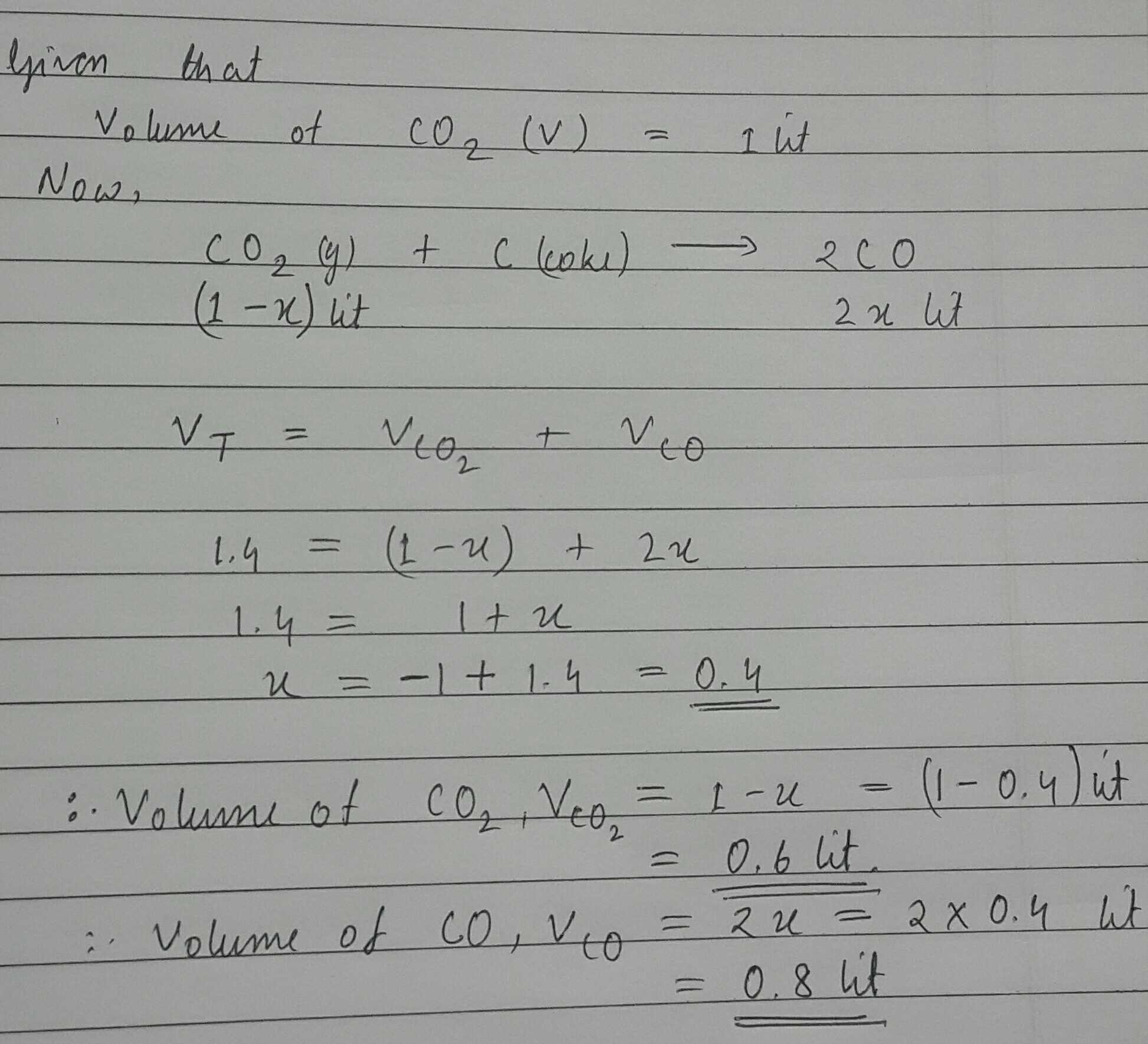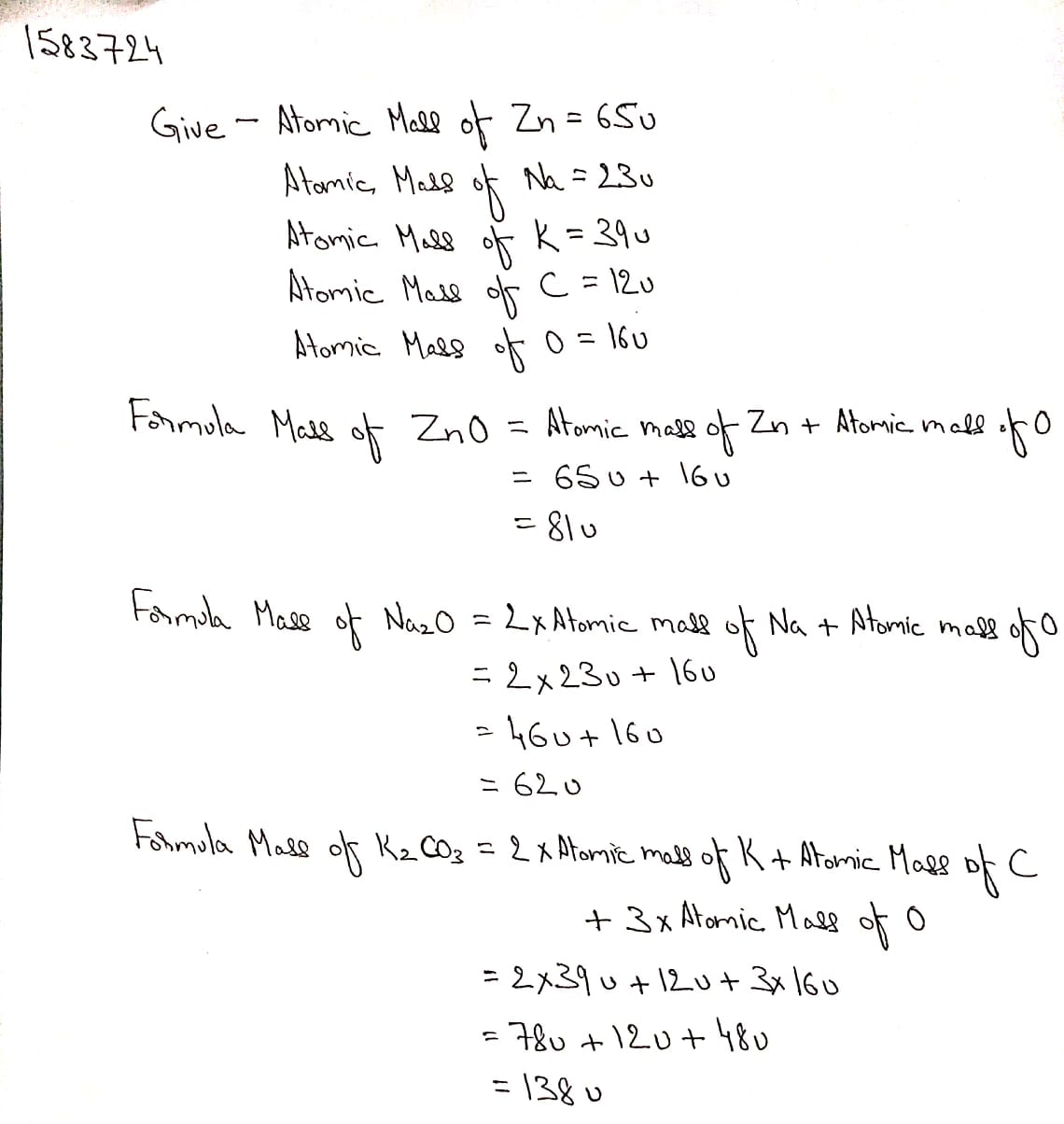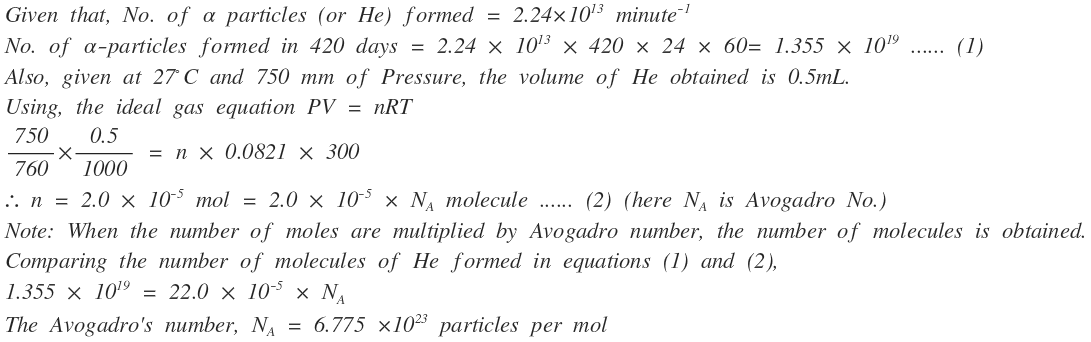Some Basic Concepts Of Chemistry - Class 11 Medical Chemistry - Extra Questions
Calculate the concentration of NaOH solution in g/mL, which has the same normality as that of a solution of HCl of concentration 0.04 g/mL.
Silver crystallises in fcc lattice. If edge length of the cell is 4.07×10−8cm and density is 10.5g cm3, calculate the atomic mass of silver.
H2SO4 is 98% by weight of solution, Mx102. What is the value of M?
Insulin contains 3.4% sulpur calculate minimum molecular weight of insulin.
1 atmosphere means how many Pascal?
Which has the highest mass ?
(a) 50 g iron (b) 5 moles of N2
(c) 0.1 mol atom of Ag (d) 1023 atoms of carbon
The total mass of reactants and products in a reaction will always be the same. This law is known as:
The atomic mass of carbon is:
16 g of oxygen and 3 g of hydrogen are mixed and kept at 760 mm pressure and 0. The total volume occupied by the mixture will be nearly
(A) 22.4 litre
(B) 33.6 litres
(C) 448 litres
(D) 44800 ml
What is hydrogen azide?
What are the conditions for something to be called as matter?
Define Avogadro's law. Taking a suitable example prove that it is not in contradiction with Dalton's Atomic Theory.
The percentage of 3 element are as follows in calcium carbonate (CaCO3) Calcium 40%, carbon 12%, Oxygen 48%. if law of constant proportion is true what weight of these element will be present in 1.5g of another sample of calcium carbonates (CaCO3)
An oxide M2O3 contains 10.3 % oxygen by weight. Calculate the atomic weight of M.
A solution contains 50 g of sugar in 350 g of water. Calculate the concentration of solution in terms of mass by mass percent of the solution.
Chlorine occurs in nature in two isotope forms with masses 35u and 37u. The percentage of 35Cl is 75%. Find the average atomic masses of chlorine atoms.
Calculate the mass of 0.125 mol NaOH
What is one amu or one 'u'?
Define atomic mass unit.
Define the atomic mass unit.
Calculate the weight of ammonia gas required for reacting with sulphuric acid to give 78 g of fertilizer ammonium sulphate.
The following questions are about one mole of sulphuric acid [H2SO4].
find the number of gram hydrogen atoms in 1 mole of [H2SO4].
During an experiment the students were asked to prepare a 10% solution of sugar in water. Ramesh dissolved 10g of sugar in 100g of water while Sarika prepared it by dissolving 10g of sugar in water to make 100g of the solution. Compare the mass % of the two solutions.
Give reason: Carbonate and sulphide ores are usually converted into oxides during the process of extraction.
The unified atomic mass unit is the standard unit that is used for indicating mass on an atomic or molecular scale. If true enter 1, else enter 0
Mass and percentage by weight do not change with ________ (temperature/volume).
The solubility of salt at 30∘C isCalculate the weight (g) of water required to prepare a saturated solution containing 90 g of salt.
If 11 g of oxalic acid is dissolved in 500 mL of solution (density = 1.1 g mL−1), what is the mass % of oxalic acid in solution?
Isotopeper Natural abundanceMolar mass1H99.98512H0.0152
The average atomic mass of hydrogen using the following data is _________.
The atomic mass of 2311Na in amu is :
The percent composition by mass of the phosphorus in calcium phosphate Ca3(PO4)2 is x. Then, the value of x is :
Boron has two stable isotopes, 10B(19%) and 11B(81%). The atomic mass that should appear for boron in the periodic table is ______ .
(Enter the nearest integer value)
The aluminum sulphate hydrate [Al2(SO4)3⋅xH2O] contains 8.20 percent Al by mass. The value of x, that is, the number of water molecules associated with each Al2(SO4)3 units is ________. (write the ans in the form of x/3)
Which of the following pairs have equal mass?
(1) 1 amu of 12C and 1 amu of 13C
(2) 1 mol of 2311Na and 1 mol of 2411Na
(3) 0.5 mol of 42He and 1 mol of 21HAnswer must be the sequence number of the pair.
Al2(SO4)3.xH2O has 8.20% Al by mass. The value of x/3 is _________.
Atomic mass unit is also called Dalton(Da). Explain.
Analysis of a metal chloride XCl3 shows that it contains 67.2% Cl by mass. The molar mass of X is __________.
Disilane, Si2Hx, is analyzed and found to contain 90.28% of silicon by weight, then the value for x is (Si=28) :
Na2SO3⋅yH2O has 50% H2O by mass. Thus, y is :
Match % of carbon in List-I with the compound given in List-II.
When 2.495 g of CuSO4⋅xH2O (molar mass 249.5) is heated, 0.05 mole of H2O is lost. Thus, x is:
A solution _______ % by mass of solvent is 16.67% by mass of solution. Write the value in the form of x×10, where x is:
Total number of ions in aluminium phosphate is :
There are_______moles of anion in one mole of sodium sulphate.
If at a particular temperature, the density of 18 M H2SO4 is 1.8 g cm−3. Percentage concentration (%) by mass of solution is :
Matter is neither .......... nor .......... in a chemical reaction.
Calculate the mass percent of O present in sodium sulphate (Na2SO4).
In diammonium phosphate, (NH4)2HPO4, the percentage of P2O5 is
Describe law of conservation of matter.
The mass of 94.5 ml of a gas at S.T.P. is found to be 0.2231 g. Calculate its molecular mass.
16 grams of NaOH is dissolved in 100 grams of water at 25oC to form a saturated solution. Find the solubility and weight percentage of the solution.
Compounds are formed by chemically combining elements in a .................... proportion by weight.
Why do atomic masses of most of the elements in atomic mass units involve fraction?
If 10l of oxygen contains x molecules, then 10l of ozone contains ______ molecules at the same temperature and pressure.
Calculate the mass of urea NH2CONH2 required in making 2.5 kg of 0.25 molal aqueous solution.
Fill in the blanks with suitable words:
The atomic mass of an atom is 23 and its atomic number is 11. The atom has ________ neutrons.
Fill in the blanks with suitable words.
Average atomic mass of chlorine is ____________.
In the brown ring complex, the oxidation state of iron is:
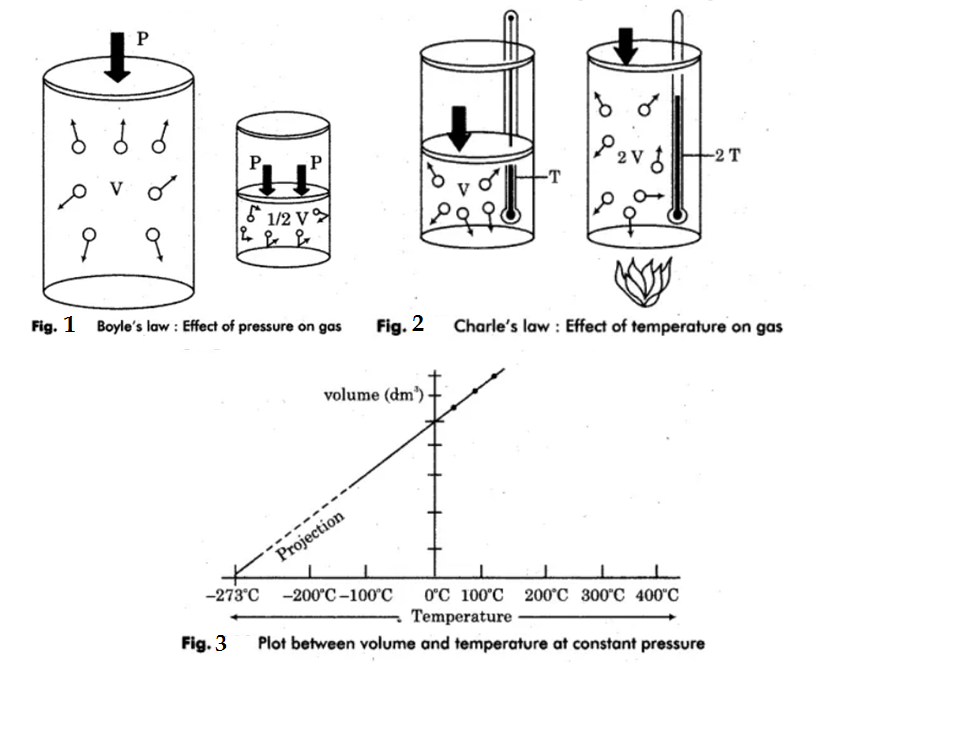
State Avogadro's law?
a) State any three findings of modem atomic theory.
b) Write down any two applications of Avogadro's law.
Find the concentration of solution in terms of weight percent if 20 grams of common salt is dissolved in 50 grams of water.
Details of some gases taken at 273 K temperature and 1 atm pressure are given in the table.
| Gas | Volume (L) | Number of moles |
| Hydrogen | 224 | 10 |
| Helium | 112 | 5 |
| Oxygen | 224 | 10 |
| Ammonia | 56 | 2.5 |
(b) Represent this gas law in mathematical form.
(c) Among these, if the pressure of hydrogen gas is changed to 2 atm. Calculate the new volume.
Find the concentration of solution in terms of weight percent if 20g of common salt is dissolved in 50g of water.
Calculate the weight of lime (CaO) that can be obtained by heating 200 kg of limestone which is 93% pure. How many moles of impure potassium chlorate of 75% purity is required to produce 48 g of oxygen?
The relative density of a mixture of nitrogen and oxygen is 14.4 (H = 1) and the relative densities of nitrogen and oxygen are 14.0 and 16.0 (H =1)respectively. Calculate the composition of the mixture (i) by volume and (ii) by mass.
2 g mixture of glucose and sucrose is dissolved in 1 litre water at 298 K to develop an osmotic pressure of 0.207 atm. Calculate percentage composition of glucose and sucrose by mole as well as by mass.
Find the amount of zinc in gram necessary to produce 224ml of H2 by the reaction with sulphuric acid.
A 0.24 g sample of compound of oxygen and boron was found by analysis to contain 0.096 g of boron and 0.144 g of oxygen.Calculate the percentage composition of the compound by weight.
Calculate the weight of lime (CaO) obtained by heating 200kg of 95% pure lime stone (CaCO3).
CaCO3→CaO+CO2
CaCO3→CaO+CO2
The density of iron crystal is 8.54 gram cm−3. If the edge length of unit cell is 2.8 Ao and atomic mass is 56 gram mol−1, find the number of atoms in the unit cell. (Given: Avogadro's number =6.022×1023, |Ao=1×10−8cm).
At 50C, liquid NH3 has ionic product is 10−30. How many amide (NH2−) ions are present per mm3 in pure liquid NH3 ? (take NA=6× 1023)
The self ionisation constant for HCOOH is 10 what percentage of HCOOH are converted to HCOO ion. The density of HCOOH is 1.22g mL−1.
Lithium exists in nature in the form of two isotopes, Li-6 and Li-7 with atomic masses 6.0151u and 7.0160u and the percentages 8.24 and 91.76 respectively. Calculate average atomic mass.
What should be the number of H + ions in 1 mL of distilled water, if the number of H + ions in L is 6.023×1016?
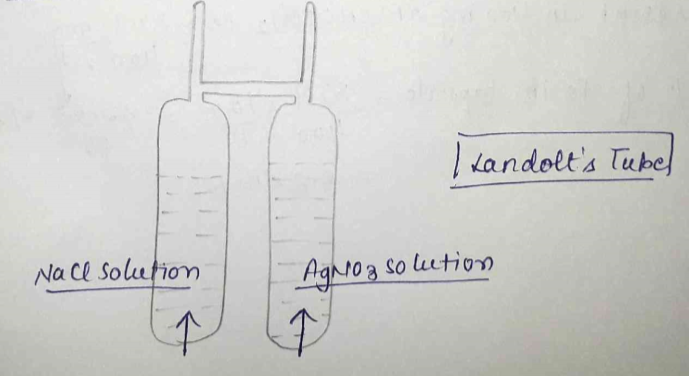
State the law of conservation of mass. State the main points of Landolt's experiment for experimental evidence of the law.
Discuss about determining mass percentage of oil from oil seed.
Calculate the percentage loss of mass of hydrated copper[II] sulphate [CuSO_4.5H_2O] when it is completely dehydrated. CuSO_4.5H_2O \to CuSO_4+5H_2O
[Atomic weights are Cu = 64, S=32, O=16, H=1]
[Atomic weights are Cu = 64, S=32, O=16, H=1]
92 g mixture of CaCO_3 and MgCO_3 heated strongly in an open vessel. After complete decomposition of the carbonates it was found that the weight of residue left behind is 48 g. Find the mass of MgCO_3 in grams in the mixture.
Calculate the mass per cent of different elements present in sodium sulphate (Na_2SO_4).
Claculate the mass % of different element in Na_2SO_4 ?
In a Carius determination, 0.234g of an organic substance gave 0.334g of barium sulphate. Calculate the percentage of sulphur in the given compound. [Ba = 137, S=32, O=16]
Calculate the no. of atoms in:a) 0.5 mol atom of nitrogenb) 0.2 mol molecules of hydrogenc)3.2 g of sulphur (Z for sulphur=32)
State and explain law of conservation of mass.
A solution contains 40 grams of common salt dissolved in 345 grams the solution. Calculate the concentration percentage of the solution by mass percentage.
0.246 g of an organic compound on complete combustion gave 0.198 g of carbondioxide and 0.1014 g of water, then the percentage composition of carbon and hydrogen in the compound respectively
1) 4.58, 21.95 2) 21.95, 4.58
3) 45.8, 2.195 4) 2.195, 45.8
State and explain laws of chemical combination.
If atomic eq. 40% of PCl_{ 5 } dissociated. Calculate equivalent molar mass.
Calculate the mass percentage composition of the elements in nitric acid :(H=1;N=14,O=16)
What is the law of conservation of mass?
Calculate the total number of electrons present in 18\ mL of water.
Explain the need for a reference atom for atomic mass. Give some information about two reference atoms.
1.20g sample of {Na}_{2}{CO}_{3} and {K}_{2}{CO}_{3} was dissolved in water to form 100ml of a solution. 20ml of this solution required 40ml of 0.1N HCl for complete neutralization. Calculate the weight of {Na}_{2}{CO}_{3} in the mixture.
How much amount of CaCO_3 in gram having percentage purity 50 per cent produces 0.56 litre of CO_2 at on heating?
Lithium exists in nature in the form of two isotopes, Li-6 and Li-7 with atomic masses 6.0151u and 7.0160u and the percentage 8.24 and 91.76 respectively.
Calculate average atomic mass.
Calculate the mass percentage composition of the elements in nitric acid (H = 1, N = 14, O = 16).
Write any two differences between mixture and compound. Also write two examples of each.
The half life of the nuclide Rn^{220} is 54.5 sec. Find mass (in kg) of radon is equivalent to 1 millicurie?
Calculate the amount of glucose required to prepare 250g of 5\% solution of glucose by mass.
4.6 cm^3 of methyl alcohol is dissolved in 25.2g of water. Calculate \% by mass of methyl alcohol. (Given density of methyl alcohol = 0.7952 gcm^{-3} and C = 12, H = 1, O = 16)
What is the mass of the precipitate formed when 50mL of 16.9\% (w/v) solution of AgNO_3 is mixed with 50mL of 5.8\% (w/v) NaCl solution?
[Ag = 107.8, N = 14, O = 16, Na = 23, Cl = 35.5]
An atom bomb is weighing 1 kg explodes releasing 9 \times 10^{13} joule of energy. What percentage of mass is converted into energy?
Calculate the mass percent of different elements in calcium phosphate.
Calculate the percentage composition in terms of mass of a
solution obtained by mixing 300 g of a 25% and 400 g of a 40% solution by mass.
Phosphoric
acid is widely used in carbonated beverages , detergents , toothpastes and
fertilizers . Calculate the mass percentage of H , P AND O in phosphoric acid
if atomis mases are H =1 , P=31 and O = 16
Calculate the mass percentage of different constituent elements in urea.
Rahul Sharma, an intelligent student of class 12 follows the lesson taught in class easily, has friendship with Amit and Krishan who do not follow the lessons easily. Rahul helps Amit and Krishan to understand the concepts and in turn, they are thankful to him. Their friendship is long lasting because both are happy. Rahul feels sense of achievement.
a) Should intelligent students make friends with weak students?Give reason.
b) If you compare this kind of friendship to atoms what kind of bond will be formed.
c) Comment on the strength of such bonding. Name the force that keeps them bonded.
d) Discuss any one property in which these atoms are different from each other.
A solution is prepared by dissolving 5 g urea in 95 g water. What is the mass percent urea in the solution?
In a gaseous mixture 2mol of { CO }_{ 2 }, 1 mol of { H }_{ 2 } and 2 mol of He are present than determine mole percentage of { CO }_{ 2 }.
At -50^0C, liquid NH_3 has ionic product is 10^{-30}. How many amide (NH_2^-) ions are present per mm^3 in pure liquid NH_3? (take N_A = 6 \times 10^{23})
What weight of zinc would be required to produce enough hydrogen to reduce 8.5 g of copper oxide completely into copper?
Calculate the mass of potassium chlorate required to liberate 6.72 of oxygen at STP.[Assume: molar mass of potassium chlorate as 122.5 g / mol].
Name the purest form of commercial ion.
44.8 L of CO_{2} at NTP is obtained by heating x g of pure CaCO_{3}.x is:
By the reaction of carbon and oxygen, a mixture of CO and CO_{2} is obtained. What is the composition(% by mass) of the mixture obtained when 20 grams of O_{2} reacts with 12 grams of carbon ?
Show that ''Law of conservation of mass is fully justified in a balanced chemical equation''.
A solution of ethanol in water is 10% by volume. If the solution and pure ethanol have densities of 0.9866\ g/cc and 0.785\ g/cc respectively, find the percent by weight.
If the atomic mass of sodium is 23u, what will be the atomic mass of sodium ion?
15.9 \,g of copper sulphate and 10.6 \,g of sodium carbonate react together to give 14.2 \,g of sodium sulphate and 12.3\,g of copper carbonate. Which law of chemical combination is obeyed? How?
Calculate the percentage loss of mass of hydrated copper [II] sulphate [ Cu{ SO }_{ 4 }] when it is completely dehydrated.
[Cu{ SO }_{ 4 }\cdot 5{ H }_{ 2 }O\rightarrow Cu{ SO }_{ 4 }+5{ H }_{ 2 }O]
[At. wts. are Cu = 64, S = 32, O = 16, H = 1].
A solution of ethanol in water is 10% by volume. If the solution and pure ethanol have densities of 0.9866 glcc and 0.785 g/cc respectively, find the percent by weight.
The mole fraction of benzene in a solution in toluene is 0.40. Calculate the weight per cent of benzene in the solution.
Define the law of conservation of mass.
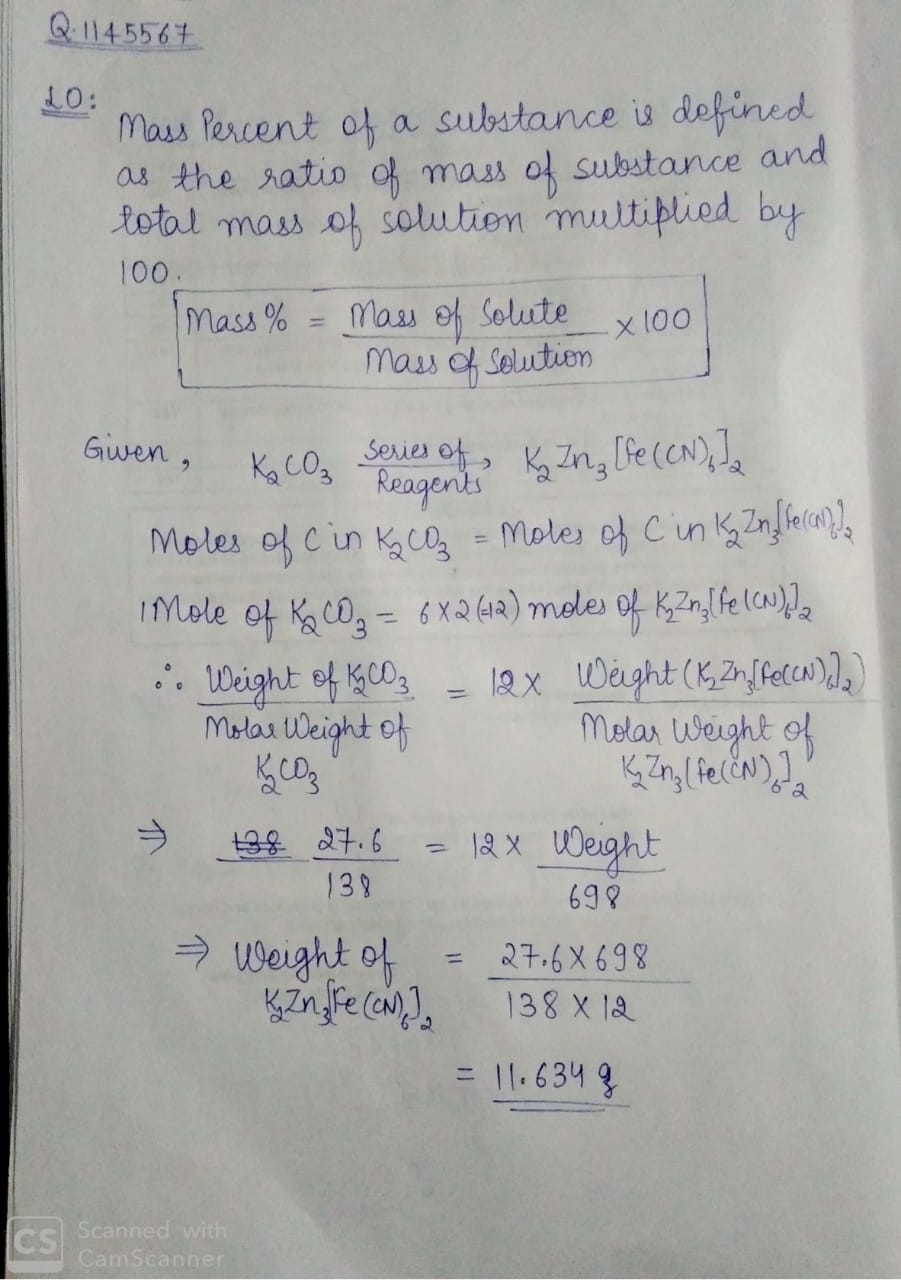
27.6 g {K_2}C{O_3} was treated by a series of reagents so as to convert all of its carbon to {K_2}Z{n_3}{\left[ {Fe{{\left( {CN} \right)}_6}} \right]_2} The weight of product is?
The percent loss in weight after heating a pure sample of potassium chlorate (mol. wt. = 122.5) will be ({KCIO }_{3 }\rightarrow KCI +{ O}_{2 })
Phosphoric acid is widely used in carbonated beverages, detergents, toothpastes and fertilizers. Calculate the mass percentages of H. P and 0 in phosphoric acid if atomis mases are H = 1, P = 31 and 0 = 16. (Ans. H - 3.06\%, P - 31.63\%, 0-65.31\%)
At STP, 2.8 liters of hydrogen sulphide were mixed with 1.6 liters of sulphur dioxide and reaction occurred according to the equation :
{ 2H }_{ 2 }S(g)+{ SO }_{ 2 }(g)\longrightarrow { 2H }_{ 2 }O(l)+3S(s)
Calculate the molality in the aqueous solution containing 3.0gm of urea in 250gm of water
(H=1,N=14,O=16)
Two samples 'm' and 'n' of slaked lime, Ca(OH)_2, were obtained from two different reactions. The details about their composition are as follows:
\underline {Sample\ 'm'} Total mass: 7 gMass of constituent oxygen: 2 gMass of constituent calcium: 5 g
\underline {Sample\ 'n'}Total mass: 1.4 gMass of constituent oxygen: 0.4 gMass of constituent calcium: 1 g
Which law of chemical combination does this prove? Explain.
Evaporation of water occurs at all temperature
How is eveporation dependent on temperature ?
Why does water vapour condense when it reaches high up in the atmosphere ?
At what temperature does water boil ?
Determine the percentage composition of each atom in the molecule of { Na } _ { 2 } { CO } _ { 3 }.
Name any two laws of chemical combination.
How to calculate the atomic mass?
Chlorine has two isotopes of atomic mass units 34.97 and 36.The relative abundance of an isotope is 0.755 and 0.245 respectively. Find the average atomic mass of chlorine.
The atomic number of an element is more important to the chemist than its relative atomic mass. Why?
The weight of 350 mL of a diatomic gas at 0^0C and 2 atm pressure is 1 g. The wt. of one atom is
a) Define atomic mass unit.
b) Distinguish between molecular mass and molar mass.
c) Give an example of (i) diatomic, (ii) triatomic molecule of compounds.
How many grans of concentrated sitric acid sol^n should be used to prepare 250 mL of 2 M HNO_3 ?
The concentrated acid is 70\% HNO_3 ?
Calcium chloride, when dissolved in water, dissociates into its ions according to the following equation :
CaCl_{ 2 (aq)} \rightarrow Ca^{2+}_{aq} +2Cl^- _{aq}
Calculate the number of ions obtained from CaCl_2 when 222 g of its dissolved in water.
Distinguish between molecular mass and molar mass.
An atom of an element is 10.1 times heavier than the mass of a carbon atom. What is its mass in a.m.u?
A gaseous mixture of H_{2} and N_{2}O gas contains 66 mass \% of N_{2}O. What is the average molecular mass of mixture?
How many amu's are present in {10^{ - 3}}g?
Express unified atomic mass unit in kg.
1 L of CO_2 is passed over hot coke.When the volume of reaction mixture becomes 1.4 L, the composition of reaction mixture is
Chlorine has two stable isotopes: Cl-35 and Cl-37 with atomic masses 34.96 and 36.95, respectively. If the average mass of chlorine is 35.43, calculate the percentage abundance.
Take 30 g of common salt and dissolve it in 70g of water. Find the concentration of a solution in terms of weight percent.
The saturation temperature for 20.7g of CuSO_4 soluble in water is_______.
Complete the table given below:
| Element | Atomic mass | Molecular mass | Atomicity number |
| Chlorine | 35.5 | 71 | - |
| Ozone | - | 48 | 3 |
| Sulphur | 32 | - | 8 |
| Nitrogen | 14 | - | 2 |
To make a saturated solution, 36 g of sodium chloride is dissolved in 100 g of water at 293 K. Find its concentration at this temperature.
Complete the following table:
| Element | Mass number | Atomic Number | number of Electrons | number of protons | number of neutrons |
| Phosphorous | 31 | 15 | |||
| Potassium | 19 | 20 |
What is the active mass of 1L of oxygen gas at N.T.P?
Calculate the formula unit masses of ZnO, Na_2O, K_2CO_3, given atomic masses of Zn = 65u, Na= 23 u, K= 39 u, C= 12 u, and O= 16 u,
\displaystyle 3.90\, g of a mixture of Al and Al_2O_3 , when reacted with a solution of sodium hydroxide, produced 840 \,mL of a gas at NTP. Find the composition of the mixture.
Radium disintegrates at an average rate of 2.24 \times 10^{13} \alpha particles per minute. Each \alpha -particle takes up two electrons from the air and becomes a neutral helium atom. After 420 days, helium gas collected was 0.5 mL, measured at 27^0 C and 750 mm Hg. Calculate the Avogadro constant.
How many years would it take to spend the Avogadro constant of rupees at the rate of 10 lakh rupees per second?
3.2\ g of a mixture of KNO_{3} and NaNO_{3} was heated to constant weight which was found to be 2.64\ g. What is the \% of KNO_{3} in mixture?
What is the weight of one atom of H in g (at. wt. of H = 1.008)?
A mixture of FeO and Fe_3O_4 when heated in air to constant weight gains 5% in its weight. Find out composition of mixture.
Does 1 g of all elements contain nucleons equal to the Avogadro constant? Explain.
Find the weight of H_2SO_4 in 1200\,mL of a solution of 0.4\,N strength.
Find the weight of NaOH in its 60 milli - equivalents.
The rate of diffusion of a sample of ozonised oxygen is 0.98 times more than that of pure oxygen. Find the percentage (by volume) of ozone in the ozonised sample. Also report percentage by weight.
Name the law of chemical combination :
(a) Which was given by Lavoisier
(b) Which was given by Proust
A mixture of NH_3(g) and N_2H_4(g) is placed in sealed container at 300 K.The total pressure is 0.5 \,atm. The container is heated to 1200 K, at which time both substances decompose completely according to the equations :
2NH_3(g) \rightarrow N_2(g) + 3H_2(g)
N_2H_4(g) \rightarrow N_2(g) + 2H_2(g)
After decomposition is complete, the total pressure at 1200\,K is found to be 4.5\,atm. Find the amount (mole) percent of N_2H_4(g) in the original mixture?
Fill in the blanks :
In water, the proportion of oxygen and hydrogen is _____ by mass.
Name the scientist who gave :
(a) Law of conservation of mass
(b) Law of constant proportions
Explain giving a suitable example:
Law of conservation of mass.
What is meant by law of conservation of mass? If 12 g of C is burnt in the presence of 32 g O_2, how much CO_2 will be formed?
Who is the father of chemistry? Why?
Calculate the average atomic mass of hydrogen using the following data:
| Isotope | \% Natural abundance | Molar mass |
| ^1 H | 99.985 | 1 |
| ^2H | 0.015 | 2 |
Calculate the mass of potassium sulphate required to prepare its 10\% solution in 100 \,g of water.
Define atomic mass.
Calculate the mass of sodium sulphate required to prepare its 20\% (mass percent) solution in 100g of water.
Calculate the mass percent of calcium, phosphorous and oxygen in calcium phosphate Ca_3(PO_4)_2.
Explain fractional atomic mass. What is the fractional mass of chlorine?
The total number of protons and neutrons in the nucleus of an atom is known as .
State the law of conservation of matter or mass.
Define law of conservation of mass for a chemical reaction.
Explain the need for a reference atom for atomic mass. Give some information about two reference atoms.
If bromine atom is available in isotopes _{ 79 }^{ 35 }{ H } (49.7 %) and _{ 81 }^{ 35 }{ H } Br (50.3 %) Calculate the average atomic mass of bromine atom.
Give reasons
Actual atomic mass is greater than mass number.
What is meant by unified mass, u?
Name the fundamental law involved in every chemical reaction.
What is Avogadro's law? Explain Boyle's law and Charle's law with graph.
Write a short note on Avogadro's Hypothesis and its applications.
Mention the signification of mole.
State Avogadro's law.
What is meant by the relative atomic mass of an element?
Calculate the relative molecular mass of carbon dioxide.
What is meant by the relative molecular mass of a substance?
Why are the atomic masses of most of the elements fractional?
Briefly explain laws of chemical combinations.
Calculate the percentage composition of carbon and oxygen in CO_2.
(Given atomic masses: Carbon = 12 and Oxygen= 16).
What do you understand from the statement that the atomic mass of Helium is 4?
One GAM substance contains Avogadro number of particles in it.
How many particles are there in Avogadro number?
One GAM substance contains Avogadro number of particles in it.
Write the number of atoms present in each of the following.
32g Oxygen
(Atomic mass S=32, O=16, C=12)
One GAM substance contains Avogadro number of particles in it.
Write the number of atoms present in each of the following.
32g Sulphur
(Atomic mass S=32, O=16, C=12)
Is their any relation between the mass of the number, if the particles are of the same mass.
One GAM substance contains Avogadro number of particles in it.
Write the number of atoms present in each of the following.
32g Carbon
(Atomic mass S=32, O=16, C=12)
Find out the mass of one mole of CaCo3. How many moles of calcium present in 1000\ g\ \ CaCo3?
What may be the method of stating the mass of atoms?
According to Avagadro's law when the temperature and pressure remain constant on which factor does the volume of gas depend?
Why are atomic mass of some elements are in fractions ?
Calculate percentage elements present in ammonium chloride (NH_4Cl)
(N=14, H=1, Cl=35.5)
What is meant by atomic mass and molecular mass?
Explain the combination capacity of an atom.
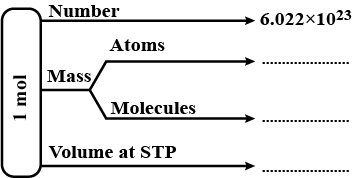
Complete the flow chart given below, related to one mole of substance.
A gas mixture of 3 \cdot 0 litres of propane and butane on complete combustion at 25^{0}C produced 10 litres of CO_{2}. Find out the composition of the gas mixture. \textbf (M.L.N.R. Allahabad\;1992)
What is kg-mole? Find out the total number of electrons in a kg-mole of O_{2}.
20 \cdot 0 mL of a mixture of oxygen (O_{2}) and ozone (O_{3}) was heated till ozone was completely decomposed. The mixture on cooling was fond to expand to 21 mL . Calculate the percentage of ozone by volume in the mixture.
Why atomic mass is an average value? Explain with a suitable example.
Why Law of conservation of mass should better be called as Law of conservation of mass and energy?
Define atomic mass, and give suitable examples.
Briefly explain the importance of studying chemistry.
Naturally occurring Boron consists of two isotopes whose atomic weights are 10 \cdot 01 and 11 \cdot The atomic weight of natural Boron is 10 \cdot Calculate the percentage of each isotope in natural Boron. \textbf{(M.L.N.R.Allahabad 1994)}
The law which states that a chemical compound always contains the same elements combined in a fixed ratio by mass is called _________.
Calcium nitrate decomposed on heating according to the equation:
2Ca(NO_{3})_{2} \rightarrow 2CaO + 4NO_{2} + O_{2}
The molecular mass of calcium nitrate is 164 u.
Calculate:
a. the volume of nitrogen dioxide (NO_{2}) obtained at S.T.P.
b. the weight of calcium oxide obtained when 16.4 g of calcium nitrate is heated to constant weight. [Ca = 40, 0 = 16, N = 14]
When 400 g of a 20% solution by weight was cooled, 60 g of solute is precipitated. The percentage concentration of the remaining solution is x \%. The value of x is _______.(Round figure the answer up to nearest integer value)
(Round figure the answer up to nearest integer value)
The percentage of copper in a copper(II) salt can be determined by using a thiosulphate titration. 0.305 gm of a copper(II) salt was dissolved in water and added to an excess of potassium iodide solution liberating iodine according to the following equation.
2Cu^{2+} (aq) + 4I^- (aq)\rightleftharpoons 2CuI(s) + I_2(aq)
The iodine liberated required 24.5 \:cm^3 of a 0.100 \:mole \:dm^{-3} solution of sodium thiosulphate
2S_2{O_{3}}^{{2-}}(aq) + I_2 (aq)\rightarrow 2I^-(aq) + S_4{O_{6}}^{{2-}}(aq)
the percentage of copper, by mass in the copper(II) salt is: [Atomic \: mass \: of \: copper = 63.5]
One type of artifical diamond (commonly called YAG for yttrium aluminium garnet) can be represented by the formula Y_{3}Al_{5}O_{12} [Y=89, Al=27]. Match element present in List-I with their weight percentages given in List-II.
A sample of clay was partially dried. It was then found to contain 50\% silica and 7\% water. The original clay contained 12\% water. Find the percentage of silica in the original sample (as nearest integer).
A mixture of CuSO_4 and CuSO_4 \, .5H_2O has a mass of 1.245 g. After heating, it drives off all the water, the mass is only 0.832 g. What is the mass (in mg) of CuSO_4.5H_2O in the mixture?
The percentage (by mass) concentration of a solution obtained by mixing 300 g of a 25% (solution I) and 400 g of a 40% (solution II) by mass is (as the nearest integer):
A solution of a specific gravity 1.6\ g\ {mL}^{-1} is 67\% by mass. The \% by mass of the solution of the same acid, if it is diluted to the specific gravity 1.2\ g\ {mL}^{-1} is (as nearest integer) :
A regular co-polymer of ethylene and vinyl chloride contains alternative monomers of each type. The mass percent of ethylene in this co-polymer is (as the nearest integer) :
Cytochrome c is an iron-containing enzyme found in the cells of all aerobic organisms. If cytochrome c is 0.43\% Fe (atomic mass = 56 amu) by weight, then the minimum molecular weight (Kg.mol^{-1}) is 6.5X. Then X is:
A polystyrene having the formula {Br}_{3}{C}_{6}{H}_{3}{({C}_{8}{H}_{8})}_{n}, was prepared by heating styrene with tribromobenzoyl peroxide in the absence of air.
If it was found to contain 10.46\% of bromine by mass, the value of n is:
When 400 g of a 20\% solution by mass was cooled, 50 g of solute precipitated. The percentage (by mass) concentration of the remaining solution is (as the nearest integer) :
To prepare 100 g of a 92% by weight solution of NaOH, how many grams of H_2O is needed?
One mole of an ideal gas at standard temperature and pressure occupies 22.4 L(molar volume). What is the ratio of molar volume to the atomic volume of a mole of hydrogen ? (Take the size of hydrogen molecule to be about 1). Why is this ratio so large ?
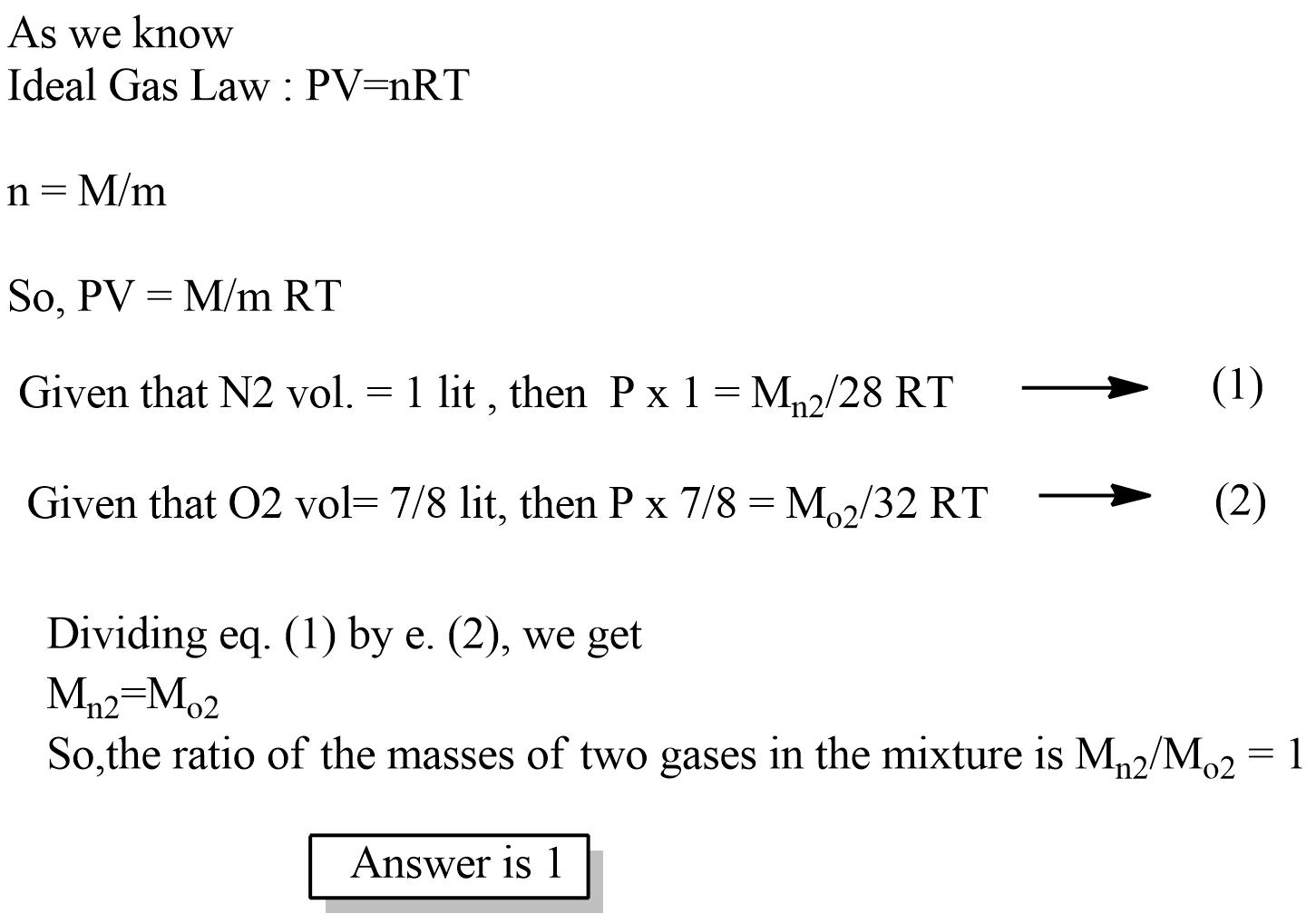
1 litre of N_2 and \cfrac{7}{8} litre of O_2 at same temperature and pressure were mixed together. The ratio of the masses of two gases in the mixture is _______.
A 0.24\ g sample of compound of oxygen and boron was found by analysis to contain 0.096\ g of boron and 0.144\ g of oxygen. Calculate the percentage composition of the compound by weight.
The relative atomic mass of an element A is 16.2 there are two isotopes _{8}^{16}A and _8^{18}A of the element. Calculate the percentage of these two isotopes present in the element.
In an experiment 1.288\ g of copper oxide was obtained from 1.03\ g of copper and 0.258\ g of oxygen. Calculate the ratio of copper and oxygen in the sample?
In a Gobar gas plant, gobar gas is formed by bacterial fermentation of animal refuse. It mainly contains methane and its heat of combustion is -809\ kJ {mol}^{-1} according to the following equation:
{ CH }_{ 4 }+2{ O }_{ 2 }\longrightarrow { CO }_{ 2 }+2{ H }_{ 2 }O;\Delta H=-809\ kJ\quad
How much gobar gas would have to be produced per day for a small village of 50 families, if it is assumed that each family requires 20,000\ kJ of energy per day? The methane content in gobar gas is 80% by mass.
One\ gram of charcoal adsorbs 100\ mL of 0.5\ M\, CH_3COOH to form a monolayer and thereby the molarity of acetic acid is reduced to 0.49\ M. Calculate the surface area of the charcoal adsorbed by each molecule of acetic acid. Surface area of charcoal = 3.01 \times 10^2\, m^2/gm.
A commercial sample (2.013 g) of NaOH containing Na_2CO_3 as an impurity was dissolved to give 250 mL of solution. A 10 mL portion of the solution required 20 mL of 0.1 N H_2SO_4 solution for complete neutralisation of NaOH. Calculate the percentage by weight of Na_2CO_3 in the sample.[Write up to 2 decimal places]
A gas cylinder can hold 1 Kg of hydrogen at mom temperature and pressure:
i) Find the number of moles of hydrogen present
ii) What weight of CO_{2} can the cylinder hold under similar conditions of temperature and pressure? (H=1,\ C=12,\ O=16)
iii) If the number of molecules of hydrogen in the cylinder is X, calculate the number of CO_{2} molecules in the cylinder under the same conditions of temperature and pressure
iv) State the law that helped you to arrive at the above result.
10g of a sample of Ba(OH)_2 is dissolved in 10mL of 0.5N HCl solution. The excess of HCl was titrated with 0.2N NaOH. The volume of NaOH used was 20cc. Calculate the percentage of Ba(OH)_2 in the sample.
Calculate the mass percentage of benzene \left( {C_6 H_6 } \right) and carbon tetrachloride \left({CCl_4}\right) if 22\ g of benzene is dissolved in 122\ g of carbon tetrachloride.
How many g of a 5.0\% by weight NaCl solution are necessary to yield 3.2g NaCl?
How many Cl atoms can you ionize in the process Cl\rightarrow {Cl}^{+}+e by the energy liberated for the process Cl+e\rightarrow {Cl}^{-} for one Avogadro number of atms. Given IP=13.0eV and EA=3.60eV. Avogadro number =6\times {10}^{23}.
An element has average atomic mass 20.426u it has 2 isotopes with masses 22.1824 and 19.9574. Calculate the abundance of its isotopes X-22 and X-20.
0.2g of an organic compound on analysis given 0.147g of carbondioxide, 0.12g of water and 74.6 c.c of nitrogen at STP. Calculate the weight percentages of constituents.
A solution contains 40 g of common salt in 320 g of water. Calculate the concentration in terms of mass by mass percentage of the solution.
Which would be larger : an atomic mass unit based on the current standard or one based on the mass of a Be- 9 atom set at exactly 9 amu ?
Density of dry air (only N_{2} & O_{2}) is 1.146\ g\ litre^{-1} at 740\ mm and 300 K. Calculate composition of air by weight assuming ideal nature.
Compute the number of ions present in 5.85 g of sodium chloride.
A sample of lead weighing 1.05g was dissolved in a small quantity of nitric acid to produce an aqueous solution of {Pb}^{2+} and {Ag}^{+} (which is present as impurity). The volume of the solution was increased to 300ml by adding water, a pure silver electrode was immersed in the solution and the potential difference between this electrode and a standard hydrogen electrode was found to be 0.503V at {25}^{o}C. What was the % of Ag in the lead metal? (Given : { E }_{ ({ Ag }^{ + }/Ag) }^{ o }=0.799V. Neglect amount of {Ag}^{+} converted to Ag)
Average atomic weight of an element M is 51.7. If two isotopes of M,\ M^{50} and M^{52} are present, then calculate the percentage of occurrence of M^{50} in nature.
The balanced equation for a reaction is given below
2X + 3Y \to {\text{4I + m}}
When 8 moles of x react with 15 moles of y, then
i) Which is the limiting reagent?
ii) Calculate the amount of products formed.
iii) Calculate the amount of excess reactant left at the end of the reaction.
Samples of O_2, N_2 and CO_2 under the same conditions of temperature & pressure contain the same number of molecules represented by X. The molecules of oxygen occupy V litres and have a mass of 8\ g. Under the same conditions of temp and press., what is the volume occupied by:
i) X molecule of N_2
ii) 3X molecules of CO_2
iii) What is the mass of CO_2 in grams.
iv) In answering the above questions, whose law has been used.
A mixture of CO of CO_2 is found to have a density of 1.5gm/ L at 30^oC & 730 torr What is the composition of the mixture.
One of the methods of preparation of per disulphuric acid, H_2S_2O_8 involve electrolytic oxidation of H_2SO_4 at anode (2H_2SO_4 \rightarrow H_2S_2O_8 +2H^+ +2e^-)
with oxygen and hydrogen as by-products. In such an electrolysis, 9.722 L of H_2 and 2.35 L of O_2 were generated at STP. What is the weight of H_2S_2O_8 formed ?
In a aqueous solution of urea, the mole fration of urea is 0.2. Calculate the mass \% of solute
A mixture of FeO and Fe_{3}O_{4} when heated in air to a constant weight gains 5\% in its weight. Find the composition of initial mixture.
A mixture containing NaHCO_{3} and Na_{2}CO_{3} weighed 1.0235\ g. The dissolved mixture was reacted with excess of Ba(OH)_{2} to form 2.1028\ g\ BaCO_{3} by the reactions;
Na_{2}CO_{3}+Ba(OH)_{2}\rightarrow BaCO_{3}+2NaOH
NaHCO_{3}+Ba(OH)_{2}\rightarrow BaCO_{3}+NaOH+H_{2}O
What is the percentage of NaHCO_{3} in the mixture?[Ba=137]
A 2.0\ g of sample containing Na_{2}CO_{3} and NaHCO_{3} loses 0.248\ g when heated to 300^{o}C. What is the percentage of Na_{2}CO_{3} in the mixture.
Write your answer to the nearest integer.
A precipitate of AgCl and AgBr weighs 0.4066\,g. On heating in a current of chlorine, the AgBr is converted to AgCl and the mixture loses 0.0725\,g in weight. Find the \% of Cl in origijnal mixture.
The total pressure of a mixture of H_2 and O_2 is 1.0 bar. The mixture is allowed to react to form water, which is completely removed to leave only pure H_2 at a pressure of 0.35 bar. Assuming ideal gas behaviour, and that all pressure measurements were made under the same temperature and volume conditions, calculate the composition of the original mixture.
A 5.0\,g sample of a natural gas consisting of CH_4, C_2H_4 was burnt in excess of oxygen yielding 14.5\,g\,CO_2 and some H_2O as product. What is weight percentage of CH_4 and C_2H_4 in mixture.
If the value of Avogadro number is 6.023 \times 10^{23} mol^{-1} and the value of Boltzmann constant is 1.380 \times 10^{-23} JK^{-1} , then the number of significant digits in the calculated value of the universal gas constant is:
A mixture containing 2.24 litres of H_2 and 1.12 litres of D_2 at NTP is taken inside a bulb connected to another bulb by a stopcock with a small opening. The second bulb is fully evacuated, the stopcock opened for a certain time and then closed. The first bulb is now found to contain 0.10\ g\ H_2. Determine the percentage composition by weight of the gases in the second bulb.
Fill in the following blank with a suitable word.Chemical equations are balanced to satisfy the law of ___________.
Enter 1 for 'conservation of mass' or 2 for 'chemical combination'.
Enter 1 for 'conservation of mass' or 2 for 'chemical combination'.
A sample of Mg metal containing some MgO as impurity was dissolved in 125\,mL of 0.1\,N\,H_2SO_4. The volume of H_2 evolved at 27.5^\circ C and 1 atm was 120.0\,mL. The resulting solution was found to be 0.02N with respect to H_2SO_4. Calculate the weight of sample dissolved and the \% by weight of pure Mg metal in sample. Neglect any charge in volume by weight of pure Mg metal in sample. Neglect any change in volume.
A plant virus is found to consist of uniform cylindrical particles of 150\overset{o}{A} in diameter and 5000\overset{o}{A} long. The specific volume of the virus is 0.75 cm^3/g. If the virus is considered to be a single particle, find its molecular weight.
Use the following data to calculate Avagodro's number(N). Density of NaCl=2.165 g cm^{-3}. Distance between Na^{+} and Cl^{-} in NaCl=281 pm.
[If x \times 10^{23} is the answer then find the value of x to the nearest integer]
A gas mixture of 3 litre of propane and butane on complete combustion at 25^\circ C produced 10 litre of CO_2. Find out the composition of mixture.
How much is 1 a.m.u?
Fill in the blanks :
The quantity of matter in an object is called its ______.
Fill in the blanks
In a chemical reaction , the sum of the masses of the reactants and the products remains unchanged . This is called _____ .
Fifty millilitre of a mixture of CO and CH_{4}, was exploded with 85 ml of O_{2}. The volume of CO_{2}, produced was 50 ml, Calculate the percentage composition of the gaseous mixture.
Why atomic masses are the average values?
Class 11 Medical Chemistry Extra Questions
- Chemical Bonding And Molecular Structure Extra Questions
- Classification Of Elements And Periodicity In Properties Extra Questions
- Environmental Chemistry Extra Questions
- Equilibrium Extra Questions
- Hydrocarbons Extra Questions
- Hydrogen Extra Questions
- Organic Chemistry Some Basic Principles And Techniques Extra Questions
- Redox Reactions Extra Questions
- Some Basic Concepts Of Chemistry Extra Questions
- States Of Matter Gases And Liquids Extra Questions
- Structure Of Atom Extra Questions
- The P-Block Elements Extra Questions
- Thermodynamics Extra Questions
- The S-Block Elements Extra Questions