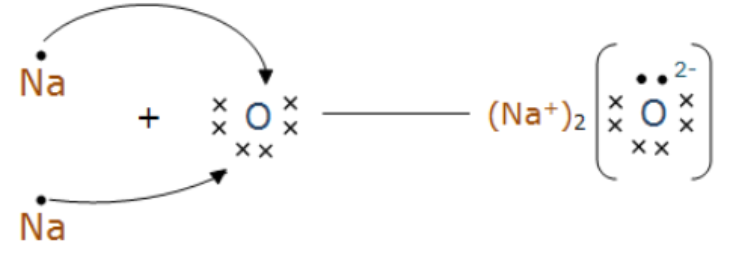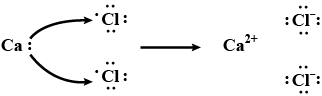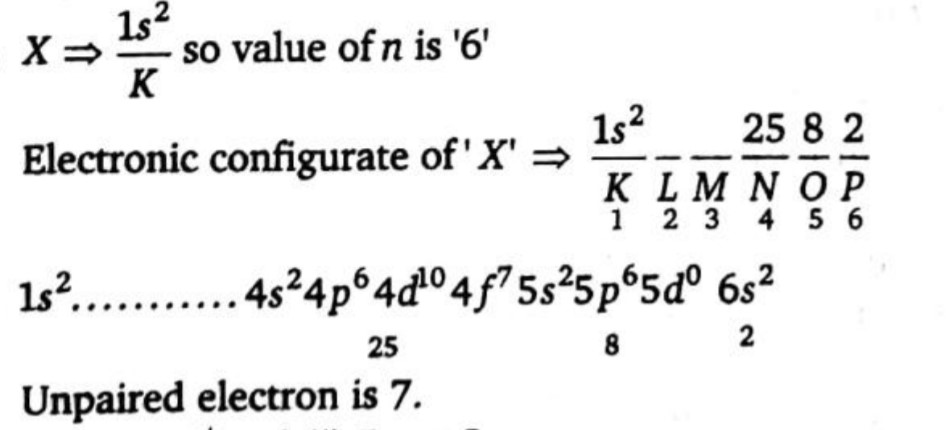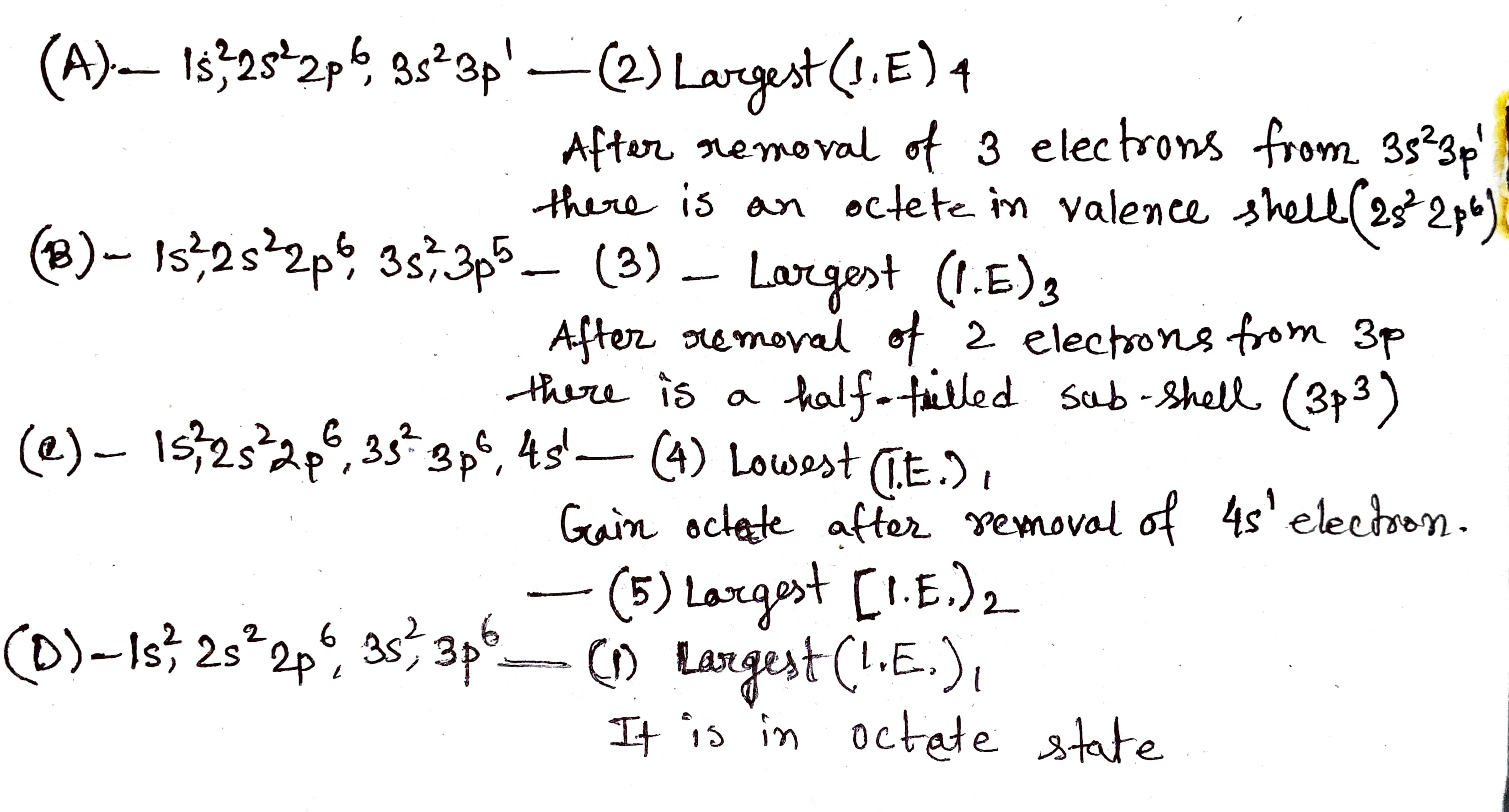Classification Of Elements And Periodicity In Properties - Class 11 Medical Chemistry - Extra Questions
A metal 'M' has the electronic configuration 2, 8, 3 and occurs in nature as M$$_2$$O$$_4$$. It is more reactive than zinc. Write a chemical equation illustrating the use of this metal for joining cracked railway lines.
____________ is the electronic configuration of the highest electronegative element.
Write the full form of IUPAC.
Complete the electronic configuration of a chloride ion.
$$1s^{2}$$ ___________.
The elements ($$z = 117$$ and $$120$$) have not yet been discovered in which family or group would you place these elements and also give electronic configuration in each case?
Name the type of elements, which have their
(i) outermost shell complete
(ii) outermost shell incomplete
(iii) two outermost shell incomplete
(iv) one electron short of octet
(v) two electrons in the outermost orbit.
What is the IUPAC name of an element with atomic number $$107$$.
With reference to periodic table, indicate:
An element that is in group IIIA and third period.
Explain chemical reactivity in periods?
Give the electronic configuration of third alkali metal.
From among the elements, choose the following:Cl, Br, F, O, Al, C, Li, Cs and Xe
The element whose atom has 6 electrons in the outermost shell.
With reference to periodic table, indicate:
The inert gases placed in the 2nd and 5th period.
Write the names of elements from the following symbols: Zn, Cd, Xe, Br, Ti, Cu, Fe, Si, Ir, Pt.
Write the electronic configuration of alkali metals.
Define the following terms.
Element
In the following table, seven elements A, B, C, D, E, F and G (here letters are not the usual symbols of the elements)
of the modern periodic table with their atomic numbers are given.
| 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| A | E | G | |||||
| 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| B | C | D | F |
In the following table, seven elements A, B, C, D, E, F and G (here letters are not the usual symbols of the elements) of the modern periodic table with their atomic numbers are given.
| 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| A | E | G | |||||
| 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| B | C | D | F |
$$\text{ With reference to period 3 of the periodic table-State}$$
The electronic configuration of the element whose hydroxide is a weak base.
In the following table, seven elements A, B, C, D, E, F and G (here letters are not the usual symbols of the elements) of the modern periodic table with their atomic numbers are given.
| 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| A | E | G | |||||
| 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| B | C | D | F |
State True or False.The atomic number of Uus is 117.
Why $$BeO$$ and $$Al_2O_3$$ are amphoteric?
B and Si resemble each other in their properties. If true enter 1, else enter 0.
$$BeCl_2$$ and $$AlCl_3$$ are Lewis acids. If true enter 1, else enter 0.
"Decreasing lattice energy favours increased solubility while decreasing hydration energy favours decreased solubility."
Answer whether the above statement is true or false.
If true enter 1, else enter 0.
As the atomic radius increases, ionisation energy is found to decrease.If true then enter 1, else enter 0.
Match the type of elements (in List I) with the corresponding electronic configuration (in List II).
IUPAC representation of the element of atomic number 115 is Uup.If true enter 1, else enter 0.
State whether the given statement is true or false:
Maximum ionization energy stands for He.
Halogen with highest ionization energy is $$F$$. State whether the statement is true or false
Find the number of element( s) which is/are present in group-I :
Be, Li, Mg, AI, Na, Ca, Cs
The number of metals from the following : $$As, Fe, Xe, Li, B, Cl, Ba, P, I, Si$$ is :
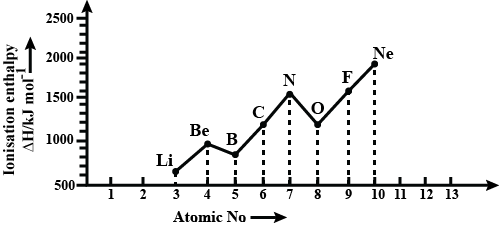
Identify the elements occupying peaks and bottommost points.
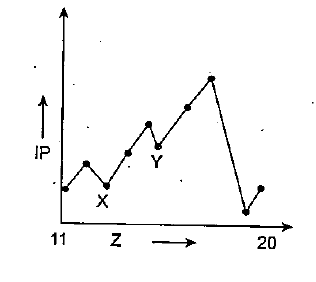
The increasing order of reactivity among group 1 element is $$Li < Na < Rb < Cs$$, whereas that among group 17 element is $$F > CI > Br > I$$. Explain?
Arrange the following in order of increasing reducing property.
$$NaH$$, $$MgH_2$$ and $$H_2O$$
$$NaH$$, $$MgH_2$$ and $$H_2O$$
Which important property did Mendeleev use to classify the elements in his Periodic Table, and did he stick to that?
What are the various factors due to which the ionization enthalpy of the main group elements tends to decrease down a group?
The first ionization enthalpy values ( in kJ mol$$ \displaystyle ^{-1} $$ ) of group 13 element are :
B Al Ga In Tl
801 577 579 558 589
How would you explain this deviation from the general trend?
801 577 579 558 589
How would you explain this deviation from the general trend?
The atomic number of elements A, B and C are (Z - 1), Z and (Z + 1) respectively. B is an inert gas (not helium). Which element among A,B and C has the highest ionization energy? Why?
In the Modern Periodic Table, calcium (atomic number $$20$$) is surrounded by elements with atomic numbers $$12, 19, 21$$ and $$38$$. Which of these have physical and chemical properties resembling calcium?
The electronic configuration of $$He$$ is $$2$$ and that of oxygen is ________.
True or False
Potassium is the most reactive alkali metals.
Which one has greater value, second ionisation energy or third ionisation energy?
Which is more metallic: sodium or aluminium?
Explain why the elements placed on the extreme left side of the periodic table acts as powerful reducing agents?
Nuclear charge increases in a period as well as group. However, periodic properties such as atomic size and IP show a reverse trend. Why?
Helium has _____configuration.
An element $$Z$$ has atomic number $$16$$. Answer the following questions on $$Z$$:
State the formula between $$Z$$ and Hydrogen?
Without knowing the electronic configurations of the atoms of elements, Mendeleev still could arrange the elements nearly close to the arrangements in the Modern Periodic Table. How can you appreciate this?
Aluminium does not react with water at room temperature but reacts with both dil. $$HCl$$ and $$NaOH$$ solutions. Verify these statements experimentally. Write your observations with chemical equations. From these observations, can we conclude that $$Al$$ is a metalloid?
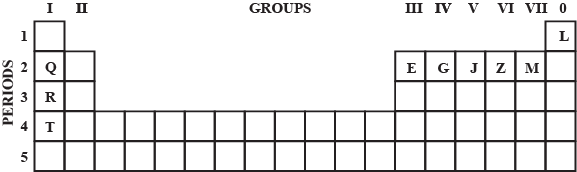
Use the letters only written in the Periodic Table given below to answer the questions that follow :
(i) State the number of valence electrons in atom $$J$$.
(ii) Which element shown forms ions with a single negative charge?
(iii) Which metallic element is more reactive than $$R$$?
(iv) Which element has its electrons arranged in four shells?
Metal $$A$$ has electronic configuration of $$2, 8, 1$$ and metal B has electronic configuration of $$2, 8, 2$$. Which metal is more reactive and why?
The element eka-silicon in Mendeleev's period table is known as ________ in the modern periodic table.
Identify the following substances which are underlined:
The element which has the highest ionization potential.
Arrange the following as per the instructions given in the brackets: $$Na, K, Cl, S, Si$$ (increasing order of ionization energy)
Complete the following Table:
| Element | Electronic configuration | Bond it forms | Valency |
| Magnesium | |||
| Oxygen | |||
| Chlorine |
In Period $$3$$ of the Periodic Table, element B is placed to the left of element A. On the basis of this information, choose the correct word from the brackets of complete the following statement.
(i) The element B would have(lower/higher) metallic character than A.
(ii) The element A would probably have(lesser/higher) electron affinity than B.
(iii) The element A would have(greater/smaller) atomic size than B.
In the modern periodic table, how does the atomic size of the elements vary along the period and down the group? Explain.
The ionisation potential value of $$K$$ is less than that of $$Na$$. Explain.
How can u predict the group, period and block of an element? Explain with examples.
In the second period, Li to Ne identify the element
(a) Most reactive element
(b) Most non-reactive non-metal
(c) Largest atomic radius
(d) With valency equal to 4
What is meant by periodicity in properties of elements with reference to the periodic table?
Why do elements in the same group have similar physical and chemical properties ?
State the appropriate scientific reasons for the following statements.
The element present in the last shell of an element decides the group in which they are placed.
Ionisation enthalpies of elements of second period are given below:
Ionisation enthalpy/$$KJ\ mol^{-1} : 520, 899, 801, 1086, 1402, 1314, 1681, 2080$$.
Match the correct enthalpy with the elements and complete the graph given in the figure. Also, write symbols of elements with their atomic number.
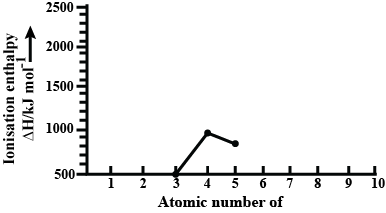
Match the correct enthalpy with the elements and complete the graph given in the figure. Also, write symbols of elements with their atomic number.
A hydrogen like atom with atomic number Z is in higher excited state of quantum number 'n' . This excited state atom can make a transition to the first excited state by successively emitting two photons of energies 10 eV and 68.2eV respectively. Alternatively the atom from the same the same excited state can make a transition to the 2nd excited state by emitting two photons of energies 4.25eV and 5.95eV respectively. Calculate the value of Z
How many electrons,protons and neutrons are present in one molecule of glucose?
An element placed in 2nd Group and 3rd Period of the Periodic Table, burns in presence of oxygen to form a basic oxide.
(a) Identify the element.
(b) Write the electronic configuration.
(c) Write a balanced equation when it burns in the presence of air.
(d) Write a balanced equation when this oxide is dissolved in water.
(e) Draw the electron dot structure for the formation of this oxide.
An element $$'X'$$ has mass number $$35$$ and number of neutrons $$18$$. Write atomic number and electronic configuration of $$'X'$$. Also write group number, period number and valency of $$'X'$$.
The second ionization enthalpy of calcium is more than that of the first and yet calcium form $${ CaCl }_{ 2 }$$ not CaCl. Why ?
$$H_2O$$ is a liquid but $$H_2S$$ is a gas. Explain why?
How does number of valency vary on moving from left to right:
in the third period of a periodic table?
Define the term ionisation enthalpy.
Among the element of the second period Li to ne pick out the element:
(i) with the highest first ionisation energy
(ii) with the highest electronegativity
Write a short note on Mendeleev's periodic law.
Four element $$P , Q , R$$ and $$S$$ belong to the third period of the Modern Periodic Table and have respectively $$1,3,5$$ and $$7$$ electrons in their outermost shells. Write the electronic configurations of $$P, Q$$ and $$R$$ and determine their valences. Write the molecular formula of the compound formed when $$P$$ and $$S$$ combine.
Arrange the following in increasing order of the property indicated:
$$ I_2,\ F_2,\ Br_2$$ and $$Cl_2$$ (reactivity)
Name the pairs showing a diagonal relationship.
Arrange the following in increasing order of the property indicated:
Na, Cu and Zn (electropositive character)
With reference to periodic table, indicate:
Position of the most electropositive element.
Explain the following:
Why is potassium a strongly electropositive element?
The element 119 has not been discovered. What would be IUPAC name and symbol for this element? On the basis of the periodic table, predict the electronic configuration of this element and also the formula of its most stable chloride and oxide.
From among the elements, choose the following:Cl, Br, F, O, Al, C, Li, Cs and Xe
The element which shows diagonal relationship with Mg.
Give the electronic configuration of ninth element of the first transition series.
Give the electronic configuration of fifth element of the first transition series.
Give the name and atomic number of the inert gas atom in which the total number of d-electrons is equal to the difference in numbers of total p and s-electrons.
The elements Na, Mg, Al, Si, P, S, Cl and Ar are arranged in the periodic table in the increasing order of their atomic numbers.
Which element has the electronic configuration?
$$ 1s^2 , 2s^2 , 2p^6 , 3s^2, 3p^1_x3p^1_y3p^1_z $$
The electronic configurations of some of the elements are given below:
(A) $$ 1s^2 , 2s^2 , 2p^6,3s^2 $$
(B) $$ 1s^2 , 2s^2 , 2p^6 $$
(C) $$ 1s^2 , 2s^2 , 2p^3 $$
(D) $$ 1s^2, 2s^2 ,2p^6,3s^1 $$
(E) $$ 1s^2 , 2s^2 , 2p^5 $$
(i) Name the elements.
(ii) Which of these has low ionisation enthalpy?
(iii) Which is a halogen?
(iv) Which is an alkali metal?
(v) Which is an inert gas?
(ii) Which of these has low ionisation enthalpy?
(iii) Which is a halogen?
(iv) Which is an alkali metal?
(v) Which is an inert gas?
Which elements have the electronic configuration $$ns^2\ np^2$$? Give their names.
An element X from group 2 reacts with element Y from group 16 of the periodic table
(a) What is the formula of the compound formed?
(b) What is the nature of the bond in the compound formed?
A metal X is in the first group of the periodic table. What will be the formula of its oxide?
Name two elements whose properties were predicted on the basis of their positions in Mendeleev's periodic table.
(a) What is the fundamental difference in the electron configurations between the group 1 and group 2 elements ?
(b) On the basis of electronic configuration how will you identify
(i) Chemically similar electrons ?
(ii) The first elements of a period ?
Atoms of eight elements A, B, C, D, E, F, G and H have the same number of electronic shells but different number of electrons in their outermost shell. It was found that elements A and G combine to form an ionic compound which can also be extracted from sea water. This compound is added in a small amount to almost all vegetable dishes during cooking. Oxides of elements A and B are basic in nature while those of E and F are acidic. The oxide of D is almost neutral. Based on the above information, Answer the following questions ;(a) To which group or period of the periodic table do these elements belong?
(b) What would be the nature of compound formed by a combination of elements B and F?
(c) Which two of these elements could definitely be metals?
(d) Which one of the eight elements is most likely to be found in gaseous state at room temperature?
(e) If the number of electrons in the outermost shell of elements C and G be 3 and 7 respectively, write the formula of the compound formed by the combination of C and G.
What property do all the elements in the same group of the periodic table as fluorine have in common
Nitrogen (atomic number 7) and phosphorus (atomic number is 15) belong to group 15 of the periodic table . Write the electronic configurations of these two elements. Which of these will be more electronegative why?
How do electronic configurations of elements change in second period of the periodic table with increase in atomic numbers?
How does the electronic configuration of an atom relate to its position in the modern periodic table?
This question refers to the elements of the periodic table with atomic numbers $$3$$ to $$18$$.
What is the electronic configuration of an element with atomic number $$10$$ ?
Study the periodic table and answer the following questions:
(i) Na has physical and chemical properties similar to which element(s) and why ?
(ii) Write the electronic configuration of N and P. Which one of these will be more electronegative and why ?
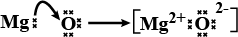
Show the formation of $$ Na_2O $$ by the transfer of electrons.
An element has electronic configuration $$2,8,7$$.
What is atomic number of this element?
Name any two elements of group one and write their electronic configurations.What similarity do you observe in their electronic configurations? Write the formula of oxide of any of the aforesaid element.
In nature, metal A is found in a free state state while metal B is found in the from of its compounds. Which of these two will be nearer to the top of the activity series of metals?
An element has electronic configuration $$2,8,7$$.
Identify the element.
In the following table are given eight elements, $$A, B, C, D, E, F, G$$ and $$H$$ (here letters are not the usual symbols of the elements) of the Modern Periodic Table with the atomic numbers of the elements in parenthesis.
| Period | Group $$1$$ | Group $$2$$ |
| $$2$$ | $$A(3)$$ | $$E(4)$$ |
| $$3$$ | $$B(11)$$ | $$F(12)$$ |
| $$4$$ | $$C(19)$$ | $$G(20)$$ |
| $$5$$ | $$D(37)$$ | $$H(38)$$ |
An element has electronic configuration $$2,8,7$$.
Name an element chemically similar to this element
Atoms of seven elements $$A, B, C, D, E, F$$ and $$G$$ have a different number of electronic shells but have the same number of electron in their outermost shell. The elements $$A$$ and $$C$$ combine with chlorine to form an acid and common salt respectively. The oxide of element $$A$$ is liquid at room temperature and is a neutral substance, while the oxide of the remaining six elements are basic in nature.
Based on the above information, answer the following question:
Show the formation of the compound by a combination of element $$C$$ with chlorine with the help of electronic structure.
Two elements $$X$$ and $$Y$$ have atomic number $$12$$ and $$16$$ respectively. To which period of the modern periodic table do these two elements belong? What type of bond will be formed between them and why? Also give the chemical formula of the compound formed.
The atomic number of Lithium is $$3$$. On the basis of this information answer the question that follows:
Write the electronic configuration of $$Li$$
In the following table, sic element $$A, B, C, D, E$$ and $$F$$ (here letter are not the usual symbols of the elements) of the modern Periodic Table with atomic numbers $$3$$ to $$18$$ are given:
| $$3$$ | $$4$$ | $$5$$ | $$6$$ | $$7$$ | $$8$$ | $$9$$ | $$10$$ |
| $$A$$ | $$E$$ | $$G$$ | |||||
| $$11$$ | $$12$$ | $$13$$ | $$14$$ | $$15$$ | $$16$$ | $$17$$ | $$18$$ |
| $$B$$ | $$C$$ | $$D$$ | $$F$$ |
Who classified the elements on the basis of fundamental properties of elements like atomic mass?
The position of eight elements in the Modern Periodic Table is given below where atomic numbers of elements are given in the parenthesis.
| Period No | ||
| 2 | Li(3) | Be(4) |
| 3. | Na(11) | Mg(12) |
| 4. | k(19) | Ca (20) |
| 5. | Rb(37) | Sr(38) |
ii) Predict the number of valence electrons in Rb
iii) What is the number of shells in Sr?
iv) Predict whether k is a metal or a non-metal.
v) Which one of these elements has the largest atom in size?
vi) Arrange Be,Ca,Mg and Rb in the increasing order of the size of their respective atoms.
The atomic number of an element 'X' is 19
a) Write its electronic configuration.
b) To which period of the Modern Periodic Table does it belong and what is its valency?
c) If 'X' burns in oxygen to form its oxide,what will be its nature-acidic,basic or neutral?
d) Write balanced chemical equation for the reaction when this oxide is dissolved in water.
Identify and name the metals out of the following elements whose electronic configurations are given below.
(a) 2, 8, 2
(b) 2, 8, 1
(c) 2, 8,7
(d) 2, 1
In Mendeleev's Periodic Table the elements were arranged in the increasing order of their atomic masses. However, cobalt with atomic mass of 58.93 amu was placed before nickel having an atomic mass of 58.71 amu. Give reason for the same.
'M' is an element which is out of Cu, Fe, Al, Na. It shows the following properties:
(i) One of its ore is rich in $$M_{2}O_{3}$$.
(ii) $$M_{2}O_{3}$$ is not affected by water.
(iii) It corrodes easily.
(iv) It forms two chlorides $$MCl_{2}$$ and $$MCl_{3}$$. Identify 'M'.
An element X which is a yellow solid at room temperature shows catenation and allotropy.X forms two oxides which are also formed during the thermal decomposition of ferrous sulphate crystals and are the major air pollutants.
a) Identify the element X
b) Write the electroic configuration of X.
c) Write the balanced chemical equation for the thermal decomposition of ferrous sulphate crystals.
d) What would be the nature (acidic/basic) of oxides formed?
e) Locate the position of the element in the Modern Periodic Table.
How do you classify elements into metals and non-metals on the basis of their electronic configuration? Choose metal and non-metal out of the following:
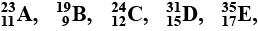
Write the formulae of chlorides of Eka-silicon and Eka-aluminium, the elements predicted by Mendeleev.
An element X (atomic number 17) reacts with an element 1' (atomic number 20) to form a divalent halide.
(a) Where in the periodic table are elements X and Y placed?
(b) Classify X and Y as metal (s), non-metal (s) or metalloid (s)
(c) What will be the nature of oxide of element Y? Identity the nature of bonding in the compound formed
(d) Draw the electron dot structure of the divalent halide
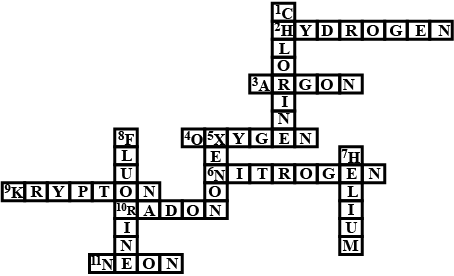
(a) In this crossword puzzle (Figure), the names of $$11$$ elements are hidden. Symbols of these elements are given below. Complete the puzzle.
(1.) Cl (2.) H (3.) Ar (4.) O (5.) Xe (6.) N (7.) He (8.) F (9.) Kr (10.) Rn (11.) Ne (b) Identify the total number of inert gases, their names, and symbols from this crossword puzzle.
Is the following symbol of element incorrect? Give the correct symbol.
Sodium So
Is the following symbol of element incorrect? Give the correct symbol.
Aluminum AL
Is the following symbol of element incorrect? Give the correct symbol.
Helium He
Mendeleev predicted the existence of certain elements not known at that time and named two of them as Eka-silicon and Eka-aluminium.
(a) Name the elements which have taken the place of these elements
(b) Mention the group and the period of these elements in the Modern Periodic Table.
(c) Classify these elements as metals, non-metals or metalloids
(d) How many valence electrons are present in each one of them?
Is the following symbol of element incorrect? Give the correct symbol.
Cobalt CO
Is the following symbol of element incorrect? Give the correct symbol.
Carbon c
Give an account of the process adopted by Mendeleev for the classification of elements. How did he arrive at "Periodic Law"?
Fill in the blanks
_________ refers to the number of atoms in the molecule of an element.
Name the scientists who discovered the following:
Elements
State the contributions of :
Dimitri Mendeleev
Fill in the blanks
The most abundant element in the earth's crust is __________.
Explain the term 'combining capacity' of an element i.e. valency.
State the trends in chemical reactivity : down the group
The element X has the electronic configuration 2, 8, 18, 8,Without identifying x,
write if X be an oxidizing agent or reducing agent and why.
How does the electronic configuration in atoms change in a group top to bottom ?
How does the electronic configuration in atoms change in a period from left to right ?
The element X has the electronic configuration 2, 8, 18, 8,Without identifying x,
predict the sigh and change on a simple ion of X.
How is sodium chloride different from its constituent elements.
Define:
(a) Elements
(b) Compounds
An element barium has atomic number $$ 56 $$ . Look up its position in the periodic table and answer the following questions :
Is it more or less reactive than calcium ?
State the trends in chemical reactivity : across the periods left to right
What is the common feature of the electronic configuration of the elements at the end of period $$ 2 $$ and period $$ 3 $$ ?
A group of elements in the periodic table are given below ( boron is the first member of the group and thallium is the last).
Boron , Aluminium , Gallium , Indium , Thallium.
Answer the following question in relation to the above group of elements :
If the electronic configuration of aluminium is $$ 2 , 8 , 3 $$ how many electrons are there in the outers shell of thallium.
The electronic configuration of nitrogen is $$(2, 5)$$. How many electrons in the outer shell of a nitrogen atom are not involved in the formation of a nitrogen molecule?
Elements X, Y and Z have atomic numbers 6, 9 and 12 respectively. Which one:
Stat type of bond between Y and Z and give its molecular formula.
How do the following change on moving from left to right in a period of the periodic table ? Give examples in support of your answer.
- Chemical reactivity of elements .
Arrange the following metals in the decreasing order of reactivity.Copper, iron, magnesium, sodium and zinc.
How does the chemical reactivity of alkali metals vary ?
Which element has :
The electronic configuration $$ 2 , 8 , 3 $$ ?
Which element from the following has the highest ionization energy ?
Explain your choice.
Ne , He , Ar
Explain why the following is not correct :
Reactivity increases with atomic number in a group as well as in a period.
Write down the electronic configuration of the : $$^{35}_{17}Y$$
Write down the electronic configuration of the following:
$$^{27}_{13}X$$
In which part of a group would you separately expect the elements to have the greatest metallic character?
From the standpoint of atomic structure, what determines which element will be the first and which the last in a period of the periodic table?
State the merits of Mendeleev's classification of elements.
From the symbol $$\dfrac {24}{12}Mg$$ state the mass number, and atomic configuration of magnesium.
This question refers to elements of the periodic table with atomic numbers from $$3$$ to $$18$$. In the table below, some elements are shown by letters, even though the letters are not the usual symbols of the elements.
$$3$$ $$4$$ $$5$$ $$6$$ $$7$$ $$8$$ $$9$$ $$10$$ $$A$$ $$B$$ $$C$$ $$D$$ $$E$$ $$F$$ $$G$$ $$H$$ $$11$$ $$12$$ $$13$$ $$14$$ $$15$$ $$16$$
$$17$$
$$18$$ $$I$$ $$J$$ $$K$$ $$L$$ $$M$$ $$N$$ $$O$$ $$P$$
What is the electronic configuration of $$G$$?
Name two elements whose atomic weights were corrected on the basis of their positions in Mendeleev's periodic table.
What are the following groups known as?
Group $$1$$
This question refers to elements of the periodic table with atomic numbers from $$3$$ to $$18$$. In the table below, some elements are shown by letters, even though the letters are not the usual symbols of the elements.
$$3$$ $$4$$ $$5$$ $$6$$ $$7$$ $$8$$ $$9$$ $$10$$ $$A$$ $$B$$ $$C$$ $$D$$ $$E$$ $$F$$ $$G$$ $$H$$ $$11$$ $$12$$ $$13$$ $$14$$ $$15$$ $$16$$
$$17$$
$$18$$ $$I$$ $$J$$ $$K$$ $$L$$ $$M$$ $$N$$ $$O$$ $$P$$
Which of these is an alkali metal?
Write electronic configuration of element $$\ ^{35}_{17}T$$
State number of protons and neutrons in $$T$$.
Name the first three alkaline earth metals.
Write their reactions with dil. hydrochloride acid.
An element $$A$$ has $$2$$ electrons in its, fourth shell. State: is it a metal or a non-metal.
An element $$A$$ has $$2$$ electrons in its fourth shell. State: its electronic configuration.
Name the alkali metals. How many electrons do they have in their outermost orbit?
An element $$A$$ has $$2$$ electrons in its fourth shell. State: is it an oxidizing or reducing agent?
Write the electronic configuration of the first two alkaline earth metals.
An element $$A$$ has $$2$$ electrons in its fourth shell. State: its atomic number.
Write electronic configuration of element $$\ ^{35}_{17}T.$$
Is it a metal or a non-metal?
Arrange the following:
Elements of group $$1$$, in increasing order of reactivity.
Write the name from the description.
The period with electrons in the shells $$K$$, $$L$$ and $$M$$
An element' X has its electronic configuration of 'K shell is $$(n - 5)s^2$$ and it has the total number of electrons in its outermost, penultimate and antepenultimate shell are $$2, 8$$ and $$25$$ respectively, then find out the total number of unpaired electrons in element 'X in their ground state.
Write down the electronic configuration of the following elements from the given atomic numbers. Answer the following question with an explanation.
$$_3Li$$, $$_{14}Si$$, $$_{11}Na$$, $$_{15}P$$
Which of these elements belong to be period $$3$$?
Write down the electronic configuration of the following elements from the given atomic numbers. Answer the following question with explanation.
$$_{1}H$$, $$_7N$$, $$_{29}Ca$$, $$_{16}S$$, $$_4Be$$, $$_{18}Ar$$.
Which of these elements belong to the second group?
Comment on the reactivity of Halogens.
Answer the following on the basis of the modern periodic table.
(I) Group no. of the elements with the valence shell ground-state electron configuration $$ns^2np^5$$
(II) Group no. of the elements with the valence shell ground-state electron configuration $$ns^2np^3$$
(Ill) Group no. of the elements that have only three unpaired p electron in the ground state
(IV) Alkaline earth metals
(V) Group 3A elements
Entries of Column-I are to be matched with entries of Column-Il. Each entry of Column-I may have the matching with one or more than one entries of Column-lI.
How do you think the tendency to lose electrons will change in a group ?
Consider the following elements A, B, C and D and their outer electronic configurations are $$ns^2np, ns^2np^3, ns^2np^4$$ and $$ns^2np^5$$ respectively. Element E also has same outer electronic configuration like D but shows only single oxidation state (-1). If element A, B, C and D belong to same period as that of sodium. Consider the following compounds.
(i) $$CE_4$$
(ii) $$BD_2E_3$$
(iii) $$DE_3$$
(iv) $$CE_2$$
(v) $$BD_3E_2$$
(vi) $$C_2E_2$$
(vii) $$DE$$
(viii) $$A_2D_6$$
Then calculate the value of "$$x \div y$$", (where x and y are total number of polar and non-polar compounds.)
The following elements(X, Y, Z) belong to same period arrange them in order of atomic radius $$\rightarrow 231 , 262, 242$$.
The electronic configuration of an atom is 2, 8,Give its atomic number and the nature of oxide.
Which element has the electronic configuration $$2, 8, 2 ?$$
How does electronic configuration of atoms change in a period with increase in atomic number ?
Why do we classify elements?
Name two elements you would expect to show chemical reactions similar to magnesium.
Name three elements with filled outermost shells.
An atom has electronic configuration 2, 8,What is the atomic number of this element?
How many shells are present in all the elements that belong to period 3?
Write the general outer electronic configurations of the following elements,
a) alkali metals
b) alkaline earth metals
c) halogens
d) noble gases
a) alkali metals
b) alkaline earth metals
c) halogens
d) noble gases
Name three elements that have two electrons in their outermost shells.
Name three elements that have a single electron in their outermost shells.
Amongst the elements $$B, Al, C\, and\,Si$$: (a) Which has the highest first ionization enthalpy? (b) Which has most negative electron gain enthalpy? (c) Which has the largest atomic radius? (d) Which has the most metallic character?
Explain the features that influence/affect the ionization energy.
(a) Account for the following: (i) $$Mg^{+2}$$ ion is smaller than $$O^{-2}$$ ion although both have same electronic structure, (ii) Ionisation enthalpy of nitrogen is more than that of oxygen,
(b) Write the IUPAC name and the symbol for the element with at.no.118.
Write the electronic configuration of $$Cl^{-}$$ ion.
Write the electronic Configuration of cation $$Fe^{+}$$.
What do you mean by electronic configuration ?
Write the electron configuration of $$Na^{+}$$ ion.
On the basis of their electronic configurations, explain why alkali metals are highly reactive.
Among the elements $$Li$$, $$K$$, $$Ca$$, $$S$$ and $$Kr$$, which one is expected to have the lowest first ionization enthalpy and which one has the highest first ionization enthalpy?
Explain electronic configuration of monoanion of N.
What is the valency of alkaline earth metal ?
Elements X is placed at first position in 15 Group . Write outer most electronic configuration of this element .
In a periodic table , the penultimate shell of which of the elements is incomplete ?
Why second ionization energy of alkali metals are higher than first ionization energy?
In what way the electronic configuration of hydrogen is similar to that of the electronic configuration of alkali metals?
Write any two factors which affect ionization.
Why alkali metals do not from di-positive ions ?
What is the IUPAC name and symbol of elements with atomic number 120 ?
What is lonization enthalpy of an element ? Explain the factors affecting it and it's periodicity?
Pick out the wrong ones fro, the subshell electronic configuration given below.
$$1s^2 2s^2 2p^5 3s^2$$
Pick out the wrong ones from, the subshell electronic configuration given below.
$$1s^2 2s^2 2p^7$$
Write electronic configuration of sodium and complete the table

Pick out the wrong ones from the subshell electronic configuration given below.
$$1s^2 2s^2 2p^6 3s^2 3p^6 3d^2 4s^2$$
Two compound of iron are given below.
$$FeSO_{4}Fe_{2}(SO_{4})_{3}$$
(The oxidation state of sulfate radical is $$-2$$)
Write the subshell electronic configuration of $$Fe^{3+}$$ ion.
Pick out the wrong ones from the subshell electronic configuration given below.
$$1s^2 2s^2 2p^2$$
Pick out the wrong ones fro, the subshell electronic configuration given below.
$$1s^2 2s^2 2p^6 3s^2 3p^6 3d^2 4s^2$$
The electronic configuration of the elements $$A, B, C, D$$ are given below.
$$A-1s^{2}\ 2s^{2}\ 2p^{6}\ 3s^{2}\ 3p^{4}$$
$$B-1s^{2}\ 2s^{2}\ 2p^{6}\ 3s^{2}$$
$$C-1s^{2}\ 2s^{2}\ 2p^{6}\ 3s^{2}\ 3p^{5}$$
$$D-1s^{2}\ 2s^{2}\ 2p^{6}\ 3s^{-1}$$
Which metal belongs to $$17^{th}$$ group ?
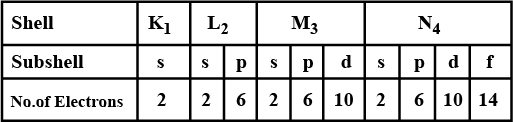
Complete the table of details about shells and subshells.
| Shell | $$K_{1}$$ | $$L_{2}$$ | $$M_{3}$$ | $$N_{4}$$ |
| Subshell | ||||
| No. of Electrons |
What is the maximum number of electrons that can be accommodated in the $$M$$ shell?
How many electrons are present ?
Identify the incorrect subshell electronic configuration.
$$-1s^{2}$$
$$-1s^{2}2p^{6}$$
$$-1s^{2}2s^{2}2p^{6}$$
$$-1s^{2}2s^{2}2p^{6}3s^{2}3p^{2}$$
The element $$Cu$$ with atomic number $$29$$ undergoes chemical reaction to formation with oxidation number $$+2$$
Write down the subshell electronic configuration of this ion.
Identify the incorrect electronic configurations and correct them.
i) $$1s^{2}\ 2s^{2}\ 2p^{3}$$
ii) $$1s^{2}\ 2s^{2}\ 2p^{6}\ 3s^{1}$$
iii) $$1s^{2}\ 2s^{2}\ 2p^{6}\ 2d^{7}$$
iv) $$1s^{2}\ 2s^{2}\ 2p^{6}\ 3s^{2}\ 3p^{6}\ 3d^{4}$$
Sub-shell electronic configuration of $$X$$ is given below. $$1s^2,2s^2,2p^5$$
Write the sub-shell electronic configuration of the element next to $$X$$ in the same period.
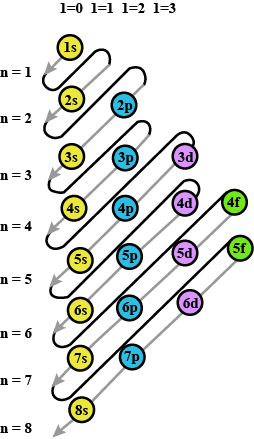
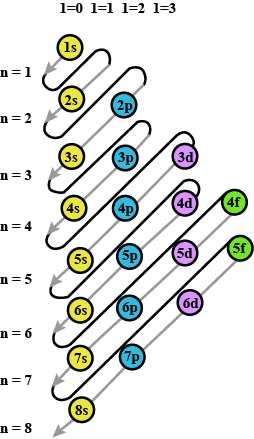
Prepare the comperhensive table which indicates the name, symbol electron configuration, subshell configuration of elements having atomic number $$1$$ to $$36$$?
Sub-shell electronic configuration of $$X$$ is given below. $$1s^2,2s^2,2p^5$$
The element $$Y$$ is coming just below the element in the same group.
Then write the sub-shell electronic configuration of $$Y$$.
Find the wrong electronic configurations from the following. What is wrong in these?
a.$$1s^2,2s^2,2p^6,3s^2,3p^6,3d^9,4s^2$$
b.$$1s^2$$
c. $$1s^2,2s^1,2p^6$$
d.$$1s^2,2s^2,2p^6,3s^2,3p^6$$
Find the wrong electronic configurations from the following. What is wrong in these?
$$1s^2$$
The atomic number of four elements are given below. (The symbols ore not real)
$$A-8$$
$$B-10$$
$$C-12$$
$$D-18$$
Write the sub-shell electronic configuration of the elements.
The atomic number of two elements are given below.
$$Si-14\ Ni-28$$
Write the subshell electronic configuration of these elements.
How many electrons are present in helium $$(_2He)$$?
The subshell electronic configuration of two elements as follows. (Symbols are not real) $$P-3s^{2}\ Q-3p^{4}$$
Write the complete subshell electronic configuration.
Match the following
| Block | Outer most electronic configuration | Characteristics |
| $$s$$ | $$3p^{5}$$ | Most of the compound are coloured. |
| $$p$$ | $$3s^{4}4s^{2}$$ | Includes Lanthanoids ($$6^{th}$$ period) |
| $$d$$ | $$4f^{1}5d^{1}6s^{2}$$ | Highest atomic radius in the respective period. |
| $$f$$ | $$3s^{1}$$ | Highelectro negativity |
In which subshell?
Complete the electronic configuration of beryllium ?
The elements $$'X'$$ has $$4$$ shells and its $$3d$$ subshell has $$6$$ electrons. (Symbol is not real)
Write the complete electronic configuration of the element.
In which shell is the electron filled?
Atomic number of iron is $$26.$$ It exhibits $$Fe^{2+},Fe^{3+}$$ oxidation state. Write the subshell electronic configuration.
| Subshell electronic configuration. | |
| $$Fe$$ $$Fe^{2+}$$ $$Fe^{3+}$$ |
Which element has the highest ionization energy?
Which element is having last electron in $$3p$$?
The outermost electronic configuration of the elements $$A$$ is $$2s^{2}\ 2p^{2}$$. (Symbol is not real)
Write the complete electronic configuration of the element just below $$A$$ in the periodic table.
Write the subshell wise electronic configu-ration of potassium .
The figure of an incomplete periodic table is given below.
Which of these elements have $$3$$ electrons in their outermost $$p$$ subshell?
Complete the table
| Element | No. of electrons | Subshells electronic configuration |
| $$_7N$$ | $$7$$ | $$1s^2\ 2s^2\ 2p^3$$ |
| $$_9F$$ | $$9$$ | $$1s^{....}2s^{.....}2p^{....}$$ |
| $$_{11}Na$$ | - | $$1s^{....}2s^{.....}2p^{....}\ 3s^{.....}$$ |
| $$_{13}Al$$ | - | $$1s^{....}2s^{.....}2p^{....} 3s^{....} 3p^{.....}$$ |
| $$_{17}Cl$$ | - | |
| $$_{18}Ar$$ | - |
Examine the given electronic configurations.
$$A-1s^{2}\ 2s^{2}\ 2p^{6}\ 3s^{2}\ 3p^{6}\ 3d^{10}\ 4s^{2}$$
$$B-1s^{2}\ 2s^{6}\ 3s^{1}$$
$$C-1s^{2}\ 2s^{2}\ 2p^{1}\ 3s^{2}\ 3p^{6}$$
$$D-1s^{2}\ 2s^{2}\ 2p^{6}\ 3s^{2}$$
$$E-1s^{2}\ 2s^{2}\ 2p^{6}\ 3s^{2}\ 3p^{6}\ 4s^{2}$$
Which element has highest metallic character?
Which element ends with electronic configuration $$4d^5,5s^1$$?
The electronic configuration of scandium $$(21Sc)$$ is
How was the shell wise electronic configuration of potassium written ?
Examine the given electronic configurations.
$$A-1s^{2}\ 2s^{2}\ 2p^{6}\ 3s^{2}\ 3p^{6}\ 3d^{10}\ 4s^{2}$$
$$B-1s^{2}\ 2s^{6}\ 3s^{1}$$
$$C-1s^{2}\ 2s^{2}\ 2p^{1}\ 3s^{2}\ 3p^{6}$$
$$D-1s^{2}\ 2s^{2}\ 2p^{6}\ 3s^{2}$$
$$E-1s^{2}\ 2s^{2}\ 2p^{6}\ 3s^{2}\ 3p^{6}\ 4s^{2}$$
Which elements belong to the same group?
Write its subshell electronic configuration of neon .
How can the subshell electronic configuration be written in a short form ?
Complete the Table
| Elements | Subshell electronic configuration |
| $$_{21}Sc$$ | $$[Ar]\ 3d^1\ 4s^2$$ |
| $$_{20}Ca$$ | ...... |
| $$_{12}Mg$$ | ...... |
| $$_{27}Co$$ | ...... |
| $$_{30}Zn$$ | ....... |
Write the subshell electronic configuration of $$_{24}Cr$$
Using the symbol of neon, write the subshell electronic configuration of sodium ?
identify the correct electronic configuration of $$29Cu$$ from those given below :$$ 1s^2\ 2s^2\ 2p^6\ 3s^2\ 3p^6\ 3d^9\ 4s^2$$$$ 1s^2\ 2s^2\ 2p^6\ 3s^2\ 3p^6\ 3d^{10}\ 4s^1$$
$$ 1s^2\ 2s^2\ 2p^6\ 3s^2\ 3p^6\ 3d^9\ 4s^2$$
$$ 1s^2\ 2s^2\ 2p^6\ 3s^2\ 3p^6\ 3d^{10}\ 4s^1$$
Subshell electronic configuration of sodium ?
Write the electronic configu ration of $$22Ti,\ 23V$$, the two elements after $$Sc$$
Electronic configuration of same elements are given. Write answers to the following questions.
i. $$[Ne]3s^2$$
ii. $$[Ar]3d^2,4s^2$$
iii. $$[Xe]6s^2$$
iv. $$[Ne]3s^2,3p^2$$
Which is the non-metal?
Write down subshell electronic configuration of $$Cu^{1+}$$ and $$Cu^{2+}$$
Write the subshell electronic configuration of the following elements and find the blocks to which they belong
$$_4Be$$
Write the subshell electronic configuration of the following elements and find the blocks to which they belong
$$26Fe$$ :
Write the subshell electronic configuration of the following elements and find the blocks to which they belong
$$18Ar$$ :
How many electrons are donated by the first group elements in a chemical reaction?
In which subshell did the filing of the last electron takes place in p block elements?
Which element shows metallic character ?
$$\ _{24}Cr-[Ar] 3d^{5}4s^{1}$$
$$\ _{29}Cu-[Ar] 3d^{10}4s^{1}$$
Why chromium and copper exhibits such electronic configuration ?
Element $$'X'$$ is having atomic number $$28,$$ it gives two electrons to element $$'Y'.$$
Write down the electronic configuration of $$'X'$$ and its ion
Write down the subshell electronic configuration of $$Fe_{21}$$.
How many electrons are donated by the second group element in a chemical reaction?
Which is the element ?
Write electronic configuration of group I elements of the periodic table.
What are the factors affecting the ionization energy?
List the elements in group $$18$$
Now try to write their electronic configuration.
Write down the complete subshell electronic configuration ?
List the elements in group $$18$$
How many electrons are there in the outermost shell of each element ?
Write the electronic configuration of elements with atomic number $$1 - 10$$
Write any two characteristics of this element ?
Which is highly reactive nonmetal ?
Why inert gases do not possess reactivity?
Find how ionization energy changes with increase in nuclear charge ?
What is doublet closed configuration? Which atom occupies this structure.
The ionisation enthalpy decreases as we go from top to bottom in the group, while it increases as we move from left to right in the period?
How many electrons are possessed by outermost orbits in the elements of $$17^{th}$$ group?
In which element except the inert gas elements have $$7$$ electrons in the outermost orbit?
What would IUPAC names and symbols for elements with atomic numbers $$122, 127 , 35 , 149 $$ and $$150$$ ?
Name the elements in the periodic table which has the highest and lowest first ionization enthalpies.
Which of the elements $$ Na , Mg , Si$$ and $$P$$ would have the greatest difference between the first and the second ionization enthalpies. Briefly explain your answer.
What would be the atomic number of the next (i) alkali metal (ii) alkaline earth metal (iii) halogen and (iv) inert gas, if discovered in future?
The values for three of the quantum numbers for the last electron in A and B are listed below. In what families of the periodic table are these elements present ?
$$l$$ $$m$$ $$s$$
A $$1$$ $$+1$$ $$+1/2$$
B $$3$$ $$-2$$ $$-1/2$$
Why ionization enthalpy of nitrogen is greater than that of oxygen ?
Explain why ionization decreases down a group of the periodic table ?
The position of certain elements in the modern Periodic Table are shown below.
$$\downarrow$$ Period /Group $$\rightarrow$$ 1 2 3 to 12 13 14 15 16 17 18 1 G H 2 A I B C 3 D E F
Using the above table answer the following questions giving reasons in each case:
Which element will form only covalent compounds?
| $$\downarrow$$ Period /Group $$\rightarrow$$ | 1 | 2 | 3 to 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| 1 | G | H | |||||||
| 2 | A | I | B | C | |||||
| 3 | D | E | F |
Using the above table answer the following questions giving reasons in each case:
Which element will form only covalent compounds?
Which element will form only covalent compounds?
In the following table, seven elements A, B, C, D, E, F and G (here letters are not the usual symbols of the elements) of the modern periodic table with their atomic numbers are given.
| 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| A | E | G | |||||
| 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| B | C | D | F |
In the following table, seven elements A, B, C, D, E, F and G (here letters are not the usual symbols of the elements) of the modern periodic table with their atomic numbers are given.
| 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| A | E | G | |||||
| 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| B | C | D | F |
Alkaline earth metals always from divalent cations even though their second ionization enthalpies are almost double than their first ionization enthalpies . Explain.
Explain the following:
Ionization enthalpy of Mg is more than that of Na and Al.
The electrons in the atoms of four elements A, B, C and D are distributed in three shells having 1, 3, 5 and 7 electrons respectively in their outermost shells. (i) Write the group numbers in which these elements are placed in the Modern
Periodic Table. (ii) Write the electronic configuration of the atoms of B and D and the molecular formula of the compound formed when B and D combine.
Periodic Table.
Write the formulae and names of the oxides of second-period elements ($$Li$$ to $$N$$). Identify the oxides as acidic, basic or amphoteric.
Which one of the following statements is incorrect in relation to ionization enthalpy?
(a) Ionization enthalpy increases for each successive electron.
(b) The greatest increases in ionization enthalpy is experienced on removal of electron from core noble gas configuration.
(c) End of valence electrons is marked by a big jump in ionization enthalpy.
(d) Removal of electron from orbitals bearing lower n value is easier than from orbital having higher n value.
Match the atomic number (in List I) with its IUPAC nomenclature (in List II).
Considering the elements F, Cl, O and N the correct order of their chemical reactivity in terms of oxidizing property is:
(a) F > Cl > O > N (b) F > O > Cl > N
(c) Cl > F > O > N (d) O > F > N > Cl
What is ionization potential? Explain the factors which affect ionization potential.
Arrange the following in the given order.
Increasing first ionization energy: $$ Mg,\ Al,\ Si, \ Na.$$
Among the elements B, Al, C, and Si,
(i) Which element has the highest first ionization enthalpy?
(ii) Which element has the most metallic character? Justify your answer in each case.
Write down the outermost electronic configuration of alkali metals. How will you justify their placement in group 1 of the periodic table?
Arrange the elements N, P, O and S in the order of :
(i) Increasing first ionization enthalpy.
(ii) Increasing non-metallic character.
Define ionization enthalpy. Discuss the factors affecting ionization enthalpy of the elements and its trends in the periodic table.
Match the column :
Match the following:
Using the part of the periodic Table given below,answer the questions that flows :
i) Na has physical and chemical properties similar to which element (s) and why?
| Groups $$\rightarrow$$ Periods $$\downarrow$$ | 1 | 2 | 13 | 14 | 15 | 16 | 17 | 18 |
| 1 | H | He | ||||||
| 2 | Li | Be | B | C | N | O | F | Ne |
| 3 | Na | Mg | Al | Si | P | S | Cl | Ar |
| 4 | K | Ca |
iii) State a chemical property common to fluorine and chlorine.
Answer the following:
Why caesium is used in photoelectric cell while lithium cannot be?
From the data given, calculate the energy deficit in the formation of $$BeCl_2$$ (it is a stable molecule).
$$I.E._1$$ for $$Be = 899\ kJ/mol$$
$$I.E._2$$ for $$Be = 1757\ kJ/mol$$
$$I.A.$$ for $$Cl = -348\ kJ/mol$$
What happens when?
Aluminium is added to copper sulphate solution.
You are given Avogadro's no. of $$ ' X ' $$ atoms . In half of the atoms of X transfer one electron to the other half of $$ ' X ' $$ atoms , $$ 409 \,kJ $$ must be added . If these $$ X^- $$ ions are subsequently converted to $$ X^+ $$ , an additional $$ 733 \,kJ $$ must be added . Calculate IP and EA of $$ X $$ in $$ eV $$ .
Use ( $$ 1 \,eV = 1.602\times10^{-19} \,J $$ and $$ N = 6.023\times10^{23} $$ ).
A mixture contains atoms of fluorine and chlorine . The removal of an electron from each atom of sample absorbs $$ 284 \,kJ $$ while the addition of an electron to each atom of mixture releases $$ 68.8\,kJ $$ .Determine the percentage composition of mixture . Given $$ IE_1 $$ for $$ F $$ and $$ Cl $$ are $$ 27.91\times 10^{-22} $$ and $$ 20.77\times 10 ^{-22} \,kJ/atom $$ respectively and $$ EA_1 $$ for $$ F $$ and $$ Cl^- $$ are $$ -5.53\times10^{-22} $$ and $$ -5.78\times10^{-22} \,kJ/atom $$ respectively .
$$ Na_{2}O $$ is basic wheres $$Cl_{2}O_{7} $$ is acidic . Explain with reason.
The ionisation enthalpy of noble gases is very high . Explain
Thin strips of magnesium, copper and iron are taken.Write down what happens when these metals are treated as follows:
Arrange these metals in decreasing order of reactivity
Match the Column $$A$$ with Column $$B$$
Which of the two elements given below would be chemically more reactive, 'X' of atomic number 18 or element 'Z' of atomic number 16 and why?
What element has the atom whose K, L, and M shells and $$4s$$ subshell are filled completely and $$4p$$ subshell is half-filled ?
Class 11 Medical Chemistry Extra Questions
- Chemical Bonding And Molecular Structure Extra Questions
- Classification Of Elements And Periodicity In Properties Extra Questions
- Environmental Chemistry Extra Questions
- Equilibrium Extra Questions
- Hydrocarbons Extra Questions
- Hydrogen Extra Questions
- Organic Chemistry Some Basic Principles And Techniques Extra Questions
- Redox Reactions Extra Questions
- Some Basic Concepts Of Chemistry Extra Questions
- States Of Matter Gases And Liquids Extra Questions
- Structure Of Atom Extra Questions
- The P-Block Elements Extra Questions
- Thermodynamics Extra Questions
- The S-Block Elements Extra Questions