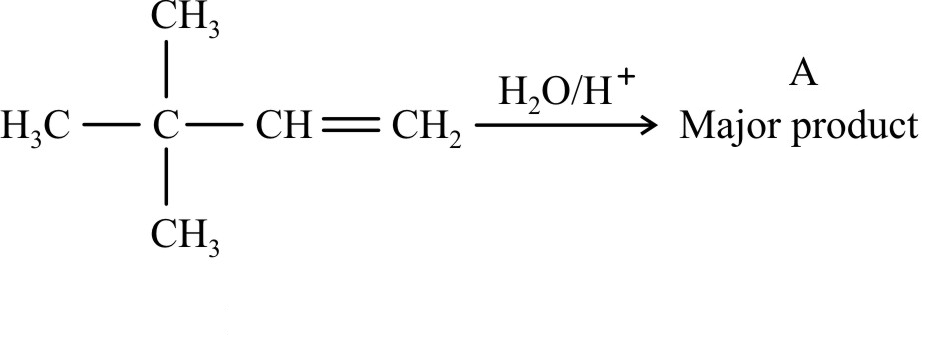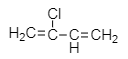NEET Chemistry MCQS - Important Questions and answers
- Alcoholsphenols Ethers MCQ Quiz
- Aldehydes Ketones Carboxylic Acids MCQ Quiz
- Amines MCQ Quiz
- Basic Concepts Chemistry MCQ Quiz
- Biomolecules MCQ Quiz
- Chemical Bonding Molecular Structure MCQ Quiz
- Chemical Kinetics MCQ Quiz
- Chemistry Everyday Life MCQ Quiz
- Classification Elements Periodicity Properties MCQ Quiz
- Coordination Compounds MCQ Quiz
- D F Block Elements MCQ Quiz
- Electrochemistry MCQ Quiz
- Environmental Chemistry MCQ Quiz
- Equilibrium MCQ Quiz
- General Principles Processes Isolation Elements MCQ Quiz
- Haloalkanes Haloarenes MCQ Quiz
- Hydrocarbons MCQ Quiz
- Hydrogen MCQ Quiz
- Organic Chemistry Basic Principles Techniques MCQ Quiz
- Pblock Elements Xi MCQ Quiz
- Pblock Elements Xii MCQ Quiz
- Polymers MCQ Quiz
- Redox Reactions MCQ Quiz
- Sblock Elements MCQ Quiz
- Solid State MCQ Quiz
- Solutions MCQ Quiz
- States Matter MCQ Quiz
- Structure Atom MCQ Quiz
- Surface Chemistry MCQ Quiz
- Thermodynamics MCQ Quiz
Alcoholsphenols Ethers MCQ Questions



Alcoholsphenols Ethers Questions and Answers
| Alcoholsphenols Ethers Quiz Question | Answer |
|---|---|
Williamson's synthesis reaction, among the following is - |
C2H5I + C2H5ONa→C2H5.O.C2H5 + NaI |
The compound that will not form a yellow precipitate on heating with an alkaline solution of iodine is: |
CH3OH |
The incorrect reaction among the following is- |
|
n-Propyl alcohol and iso-propyl alcohol can be distinguished by - |
oxidation with potassium dichromate |
The products of combustion of an aliphatic thiol (RSH) at 298K are:- |
CO2(g), H2O(g)andSO2(g) |
Among the following sets of reactants which one produces anisole? |
C6H5OH, NaOH, CH3I |
Which one is formed when sodium phenoxide is heated with ethyl iodide? |
Phenetole |
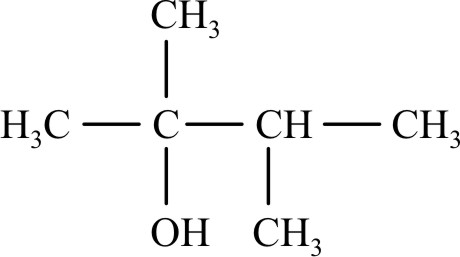
Which reagent will convert propionic acid to propanol-1? |
LiAlH4 |
Butan-2-ol is a- |
Secondary alcohol |
Primary alcohols can be obtained from the reaction of the RMgX with: |
HCHO |
Aldehydes Ketones Carboxylic Acids MCQ Questions



Aldehydes Ketones Carboxylic Acids Questions and Answers
| Aldehydes Ketones Carboxylic Acids Quiz Question | Answer |
|---|---|
Ketones are first oxidation product of: |
secondary alcohols |
Alkaline hydrolysis of R2C.Cl2 forms: |
alkanone |
Ketones are prepared by: |
Oppeanauer's oxidation |
Chlorine does not react with: |
methanal |
Both acetaldehyde and ketone react with:
|
2,4-dinitro phenylhydrazine |
Aldehydes are produces in atmosphere by: |
reaction of oxygen atoms with hydrocarbons |
Among acetic acid, phenol and n-hexanol which one of the following compound will react with NaHCO3 solution to give sodium salt and CO2? |
Acetic acid |
The reagent used for the separation of acetaldehyde from acetophenone is- |
NaHSO3 |
Iodoform test is not given by |
3-pentanone |
Which of the following compounds with molecular formula, C5H10 yields acetone on ozonolysis? |
2-methyl-2-butene |
Amines MCQ Questions



Amines Questions and Answers
| Amines Quiz Question | Answer |
|---|---|
The compound formed when malonic ester reacts with urea is: |
barbituric acid |
The type of isomerism shown by C6H5CN and C6H5NC is: |
Functional |
Compound that will undergo Hoffmann reaction is- |
RCONH2 |
The reagent needed to convert acetamide into methyl amine is - |
NaOH/ Br2 |
|
Intramolecular N-N coupling |
The hydrochlorides of amines form double salt with: |
both (a) and (b) |
Which of the following can not be formed by Gabriel phthalimide synthesis:- |
|
|
|
An amine gives a solid compound that is insoluble in alkali with benzene sulphonyl chloride. The amine is - |
CH3-NH-CH2-CH3 |
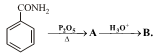
The compound A and B respectively are
|
2 |
Basic Concepts Chemistry MCQ Questions



Basic Concepts Chemistry Questions and Answers
| Basic Concepts Chemistry Quiz Question | Answer |
|---|---|
A significant figure is defined as - |
The total number of digits in a number, including the last digit that represents the uncertainty of the result. |
How many moles of lead (II) chloride will be formed from a reaction between 6.5g of PbO and 3.2g of HCl? |
0.029 |
The concentrated nitric acid is 70% HNO3. The amount of concentrated nitric acid solution that should be used to prepare 250 mL of 2.0 M HNO3 would be |
45.0 g conc. HNO3 |
What is the mole fraction of the solute in a 1.00 molal aqueous solution? |
0.0177 |
6.02x1020 molecules of urea are present in 100mL of its solution. The concentration of the solution is |
0.01M |
Which of The following has a maximum number of molecules? |
2g H2 |
The number of moles of KMnO4 reduced by one mole of KI in an alkaline medium is- |
Two |
The number of moles of oxygen in 1L of air containing 21% oxygen by volume, under standard conditions, is |
0.0093 mole |
What volume of oxygen gas (O2) measured at 0°C and 1 atm, is needed to burn completely 1L of propane gas (C3H8) measured under the same conditions? |
5L |
1 g of magnesium is burnt with 0.56g of oxygen in a closed vessel.The surplus reactant and its quantity are (At. weight of Mg = 24, O=16) |
Mg, 0.16g |
Biomolecules MCQ Questions



Biomolecules Questions and Answers
| Biomolecules Quiz Question | Answer |
|---|---|
The two forms of glucopyranose obtained from solution of D-glucose are called |
Anomers |
Sucrose on treatment with conc. HCl produces:- |
Glucose + Fructose |
During the process of digestion, the proteins present in food materials are hydrolyzed to amino acids. The two enzymes involved in the process Proteins Enzyme (A)→ Polypeptides Enzyme (B)→ Amino acids are respectively |
pepsin and trypsin |
Phospholipids are esters of glycerol with |
two carboxylic acid residues and one phosphate groups |
Compound that gives positive Fehling's solution test is : |
Glucose |
Which of the following is the sweetest sugar? |
Fructose |
a-D-(+)-glucose and b-D-(+)-glucose are |
Anomers |
The couplings between base units of DNA is through : |
Hydrogen bonding |
The segment of DNA which acts as the instrumental manual for the synthesis of the protein is |
gene |
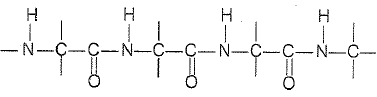
Which of the following structures represents the peptide chain ? |
|
Chemical Bonding Molecular Structure MCQ Questions



Chemical Bonding Molecular Structure Questions and Answers
| Chemical Bonding Molecular Structure Quiz Question | Answer |
|---|---|
The strongest hydrogen bond present among the following is- |
F—H‐‐‐F |
Which of the following is the most basic oxide? |
Bi2O3 |
In which of the process, the bond order increases and magnetic behaviour changes? |
() NO→NO+ |
Among the following, which has the maximum hydration energy? |
H+ |
Among the following oxides, which has the maximum lattice energy? |
MgO |
Which of the following pairs of species have similar shapes? |
PCl3, NH3 |
Incorrect statement about resonance is : |
Hybrid structure is most energetic. |
Which of the following species deviates from the octet rule? |
H2SO3 |
Which of the following species has a bent T-shape? |
BrF3 |
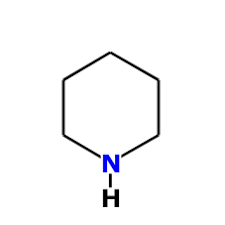
In piperidine "N" atom has hybridization:
|
sp3 |
Chemical Kinetics MCQ Questions



Chemical Kinetics Questions and Answers
| Chemical Kinetics Quiz Question | Answer | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Hydrogenation of vegetable ghee at 250C reduces pressure of H2 from 2 atom to 1.2 atom in 50 minute. The rate of reaction in terms of molarity per second is:
|
1.09 x 10-5 |
||||||||||||
A hypothetical reaction, A2 +B2 → 2AB mechanism as given below; A2 ⇌A+ A ............(Fast) A+B2 → AB + B ............(Slow) A+ B → AB ............(Fast) The order of the overall reaction is:
|
1.5 |
||||||||||||
Following mechanism has been proposed for a reaction, 2A+B →D+E A+B→ C+D ...(Slow) A+ C→ E ...(Fast) The rate law expression for the reaction is: |
r=K[A][B] |
||||||||||||
In gaseous reactions important for the understanding of the upper atmosphere H2O and O react bimolecularly to form two OH radicals. ∆H for this reaction is 72kJ at 500 K and Ea is 77 kJ mol-1, then Ea for the bimolecular recombination of two OH radicals to form H2O and O is: |
5 kJ mol-1 |
||||||||||||
For a reaction A → Product, rate law is -d[A]dt=K[A]0. The concentration of A left after time t when t=1K is: |
[A]0e |
||||||||||||
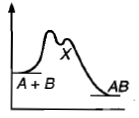
For an exothermic chemical process occurring in two steps as; (i) A+B→X(Slow) (ii) X→AB (Fast) The progress of the reaction can be best described by: |
|
||||||||||||
For the non-stoichiometric reaction 2A + B → C +D, the following kinetic data were obtained in three separate experiments, all at 298 K.
The rate law for the formation of C is: |
d[C]/dt = k[ A] |
||||||||||||
For the reaction N2 + 3H2 → 2NH3, the rate d[NH3]dt= 2 x 10-4 M s-1 .Therefore, the rate -d[N2]dt is given as: |
10-4 Ms-1 |
||||||||||||
If 'I' is the intensity of absorbed light and 'c' is the concentration of AB for the photochemical process AB + hv→ AB *, the rate of formation of AB * is directly proportional to: |
I |
||||||||||||
Which curve represents zero order reaction? |
|
Chemistry Everyday Life MCQ Questions



Chemistry Everyday Life Questions and Answers
| Chemistry Everyday Life Quiz Question | Answer |
|---|---|
Artificial sweetener that is stable under cold conditions only is : |
Aspartame |
The incorrect statement among the following is : |
Dilute solutions of boric acid and hydrogen peroxide, are strong antiseptics. |
Which one of the following is employed as antihistamine? |
Diphenyl hydramine |
Which one of the following is employed as a tranquilizer drug? |
Valium |
Which of the following protein destroy the antigen when it enters in body cell?
|
Antibodies |
Vitamin- B12 contains |
Co (II) |
Enzymes take part in a reaction and |
increase the rate of a chemical reaction |
Which one of the following vitamins is water-soluble? |
Vitamin-B |
Which one of the following is employed as a tranquiliser? |
Equanil |
Commonly used antiseptic 'Dettol' is a mixture of |
chloroxylenol + terpineol |
Classification Elements Periodicity Properties MCQ Questions



Classification Elements Periodicity Properties Questions and Answers
| Classification Elements Periodicity Properties Quiz Question | Answer |
|---|---|
Transition elements exhibit variable oxidation states because they release electrons from the following orbits:
|
(n-1)d and ns orbits |
According to Moseley, a straight-line graph is obtained on plotting- |
The square root of the frequencies of characteristic X-rays of elements against their atomic numbers |
The tenth element in the periodic table resides in: |
the second period |
Which of the following order is not in accordance with the property stated against it? |
F2 > Cl2 > Br2 > l2 Bond dissociation energy |
An element X occurs in short period having configuration ns2np1 . The formula and nature of its oxide are: |
X2O3, amphoteric |
The outermost electronic configuration of the most electronegative element is : |
ns2np5 |
The elements X, Y, Z and J have the indicated electron configurations starting with the innermost shell. The most metallic element is : |
Z = 2, 8, 8, 1 |
Which statement is true? |
The electronegative nature of elements increases along the period |
The most non-metallic element among the following is: |
B |
58Ce is a member of which block? |
f-block elements. |
Coordination Compounds MCQ Questions



Coordination Compounds Questions and Answers
| Coordination Compounds Quiz Question | Answer |
|---|---|
From the stability constant (hypothetical values)given below, predict which is the strongest ligand? |
Cu2++4CN⇌[Cu(CN)4]2-;(K=2.0x1027) |
Which does not obey EAN rule? |
[Cu(NH3)4]2+ |
Which of the following species can act as reducing agent ? |
Mn(CO)6 |
The coordination number and oxidation state of Cr in K3[Cr(C2O4)3] are respectively |
6 and +3 |
The octahedral complex will not show geometrical isomerism is : |
[MA5B] |
According to IUPAC nomenclature sodium nitroprusside is named as |
sodium pentacyanonitrosyl ferrate(II) |
The compound that is not π-bonded organometallic compound is : |
(CH3)4Sn |
IUPAC name of [Pt(NH3)3 (Br)(NO2)Cl]Cl is : |
Triamminebromidochloridonitroplatinum(IV)Chloride |
Which of the following cation does not form an amine complex with excess of ammonia? |
Na+ |
The denticity of EDTA is : |
Hexadentate. |
D F Block Elements MCQ Questions



D F Block Elements Questions and Answers
| D F Block Elements Quiz Question | Answer |
|---|---|
The colourless species among the following are : |
TiF2-6 and Cu2Cl2 |
identify the incorrect statement among the following: |
As a result of lanthanoid contraction, the properties of 4d series of the transition elements have no similarities with the 5d series of elements |
The lanthanide contraction is responsible for the fact that : |
Zr and Hf have about the same atomic radius . |
More number of oxidation states are exhibited by the actinoids than by the lanthanoids. The main reason for this is: |
Lesser energy difference between 5f and 6d orbitals than that between 4f and 5d orbitals |
The electronic configuration of the last element of actinoids is- |
Lawrencium, Lr: [Rn]5f146d17s2 |
Incorrect statement regarding the stability of oxidation state for the elements of 3d series is - |
The relative stability of the +2 oxidation state decreases on moving from top to bottom. |
In which of the following pairs are both the ions coloured in aqueous solution? (At. no : Sc =21, Ti = 22, Ni = 28, Cu = 29, Co = 27) |
Ni2+, Ti3+ |
The correct order of atomic/ionic radii is: |
Sc>Ti>V>Cr |
Which is not true in case of transition metals |
They crystallise with body centered cubic and hexagonal close packed structure only |
Which one of the following statements related to lanthanons is incorrect? |
All the lanthanons are much more reactive than aluminium |
Electrochemistry MCQ Questions



Electrochemistry Questions and Answers
| Electrochemistry Quiz Question | Answer |
|---|---|
The standard reduction potentials of Cu2+/Cu and Cu2+/Cu+ are 0.337 and 0.153V respectively. The standard electrode potential of Cu+/Cu half cell is: |
0.521V |
When a lead storage battery is discharged, then: |
Sulphuric acid is consumed. |
Metals have conductivity of the order of (ohm-1 m-1):- |
108 |
An electrochemical cell is shown below Pt, H2(1 atm)| HCI (0.1 M)CH3COOH (0.1 M)| H2(1 atm), Pt The EMF of the cell will not be zero, because 1. EMF depends on molarities of acids used |
2 |
Saturated solution of KNO3 is used to make 'salt-bridge' because: |
Velocities of both K+ and NO-3 are nearly the same |
A current is passed through two voltameters connected in series. The first voltmeter connected in series. The first voltmeter contains XSO4(aq) while the second voltmeter contains Y2SO4(aq). The relative atomic masses of X and Y are in the ratio of 2:1. The ration of the mass of X liberated to the mass of Y liberated is: |
1:1 |
The mass of silver(eq. mass = 108) displaced by that quantity of current which displaced 5600 mL of hydrogen at STP is: |
54 g |
A silver cup is plated with silver by passing 965 coulomb of electricity. The amount of Ag deposited is:
|
1.08 g |
Which is the correct representation for Nernst equation ? |
All of the above |
When a copper wire is immersed in a solution of AgNO3, the colour of solution becomes blue because copper: |
Is oxidised to Cu2+ |
Environmental Chemistry MCQ Questions



Environmental Chemistry Questions and Answers
| Environmental Chemistry Quiz Question | Answer |
|---|---|
Green chemistry means such reactions that-
|
Reduce the use and production of hazardous chemicals. |
The wrong statement about photochemical smog is- |
It has a low concentration of oxidizing agents |
A gas that is not common component of photochemical smog is- |
Chlorofluorocarbons |
Which one of the following statements regarding photochemical smog is not correct? |
Photochemical smog does not cause irritation in eyes and throat |
Which oxide of nitrogen is not a common pollutant introduced into the atmosphere both due to natural and human activity? 1. N2O52. NO23. N2O4. NO |
1 |
Which one of the following statements is not true? |
Concentration of dissolved oxygen below 5 ppm is good for the growth of fish |
Among the following, the one that is not a greenhouse gas is: |
Sulphur dioxide |
The phenomenon in which atmospheric gases trap the heat radiations from the sun near the earth’s surface and keeps it warm is known as- |
Natural greenhouse effect |
The gas that damages the ozone layer is - |
CFCs |
Biosphere is |
In which individual interact to each other |
Equilibrium MCQ Questions



Equilibrium Questions and Answers
| Equilibrium Quiz Question | Answer |
|---|---|
Which is hydrazoic acid's conjugate base? |
Azide ion |
Among the following , the compound with highest pH is : |
Na2CO3 |
The phenomenon of interaction of anions and cations furnished by an electrolyte with the H+ and OH- ions of water to produce acidic nature or alkalinity is known as hydrolysis. In hydrolysis: |
all of the above |
The degree of dissociation of PCl5 (α) obeying the equilibrium, PCl5 (g) ⇌ PCl3 (g) + Cl2 (g), is approximately related to the pressure at equilibrium by: |
α∝1/√P |
In a system: A(s) ⇌2B(g) + 3C(g). If the concentration of C at eqilibrium is increased by a factor 2, it will cause the eqilibrium concentration of B to change to: |
12√2 times of its original value |
A weak acid, HA has a Ka of 1.00 x 10-5. If 0.100 mole of this acid is dissolved in one litre of water, the percentage of acid dissociated at equibrium is closest to |
1.00% |
If little heat is added to ice ⇌ liquid equilibrium in a sealed container, then: |
No change in P and T |
In the equilibrium, 2SO2(g) + O2 (g) ⇌2SO3(g), the partial pressure of SO2, O2 and SO3 are 0.662, 0.101 and 0.331 atm respectively. What should be the partial pressure of oxygen so that the equilibrium concentration of SO2 and SO3 are equal. |
0.4 atm |
Ionisation constant of CH3COOH is 1.7 X 10-5 and concentration of H+ ions is 3.4 X 10-4.Then, find out initial concentration of CH3COOH molecules.
|
6.8 X 10-3 |
The formation of phosgene is represented as, CO + Cl2 ⇌COCl2 The reaction is carried out in 500 mL flask. At equilibrium o.3 mole of phosgene, 0.1 mole of CO and 0.1 mole of Cl2 are present. The equilibrium constant of the reaction is: |
15 |
General Principles Processes Isolation Elements MCQ Questions



General Principles Processes Isolation Elements Questions and Answers
| General Principles Processes Isolation Elements Quiz Question | Answer |
|---|---|
Chalcogens are: |
ore forming elements |
Which process is not used in purification of bauxite? |
Frankland's method |
Chloride ore among the following is: |
Karnalite |
Corundum is:
|
Al2O3 |
Heating of carbonate ores to remove carbon is called as: |
Calcination |
Sulphide ores are common for the metals: |
Ag, Cu and Pb |
Which element occurs freely in nature? |
Sulphur |
Silver obtained by argentiferrous lead is purified by: |
cupellation |
High purity copper metal is obtained by: |
Electrolytic reduction |
Aluminothermic process is used for the extraction of metals, whose oxides are: |
not easily reduced by carbon |
Haloalkanes Haloarenes MCQ Questions



Haloalkanes Haloarenes Questions and Answers
| Haloalkanes Haloarenes Quiz Question | Answer |
|---|---|
When CH3CH2CHCl2 is treated with NaNH2, the product formed is |
CH3-C≡CH |
Chlorobenzene reacts with Mg in dry ether to give a compound (A), |
Benzene |
R-CH2-CCl2-R Reagent→R-C≡C-R. The reagent is |
KOH in C2H5OH |
Ethyl chloride is converted into diethyl ether by - |
Williamson's synthesis |
The alkyl halide is converted into an alcohol by |
Substitution |
The addition of Br2 on cis-but-2-ene gives: |
Racemic mixture of 2,3-Dibromobutane |
The number of different substitution products possible when ethane is allowed to react with bromine in sunlight are: |
9 |
|
racemic mixture |
The reactivity order of halides for dehydrohalogenation is |
R-I>R-Br>R-Cl>R-F |
In SN2 reactions, the correct order of reactivity for the following compounds : CH3Cl, CH3CH2Cl, (CH3)2CHCl and (CH3)3CCl is : |
CH3Cl>CH3CH2Cl>(CH3)2CHCl>(CH3)3CCl |
Hydrocarbons MCQ Questions



Hydrocarbons Questions and Answers
| Hydrocarbons Quiz Question | Answer |
|---|---|
Which of the following reagents will be able to distinguish between 1-butyne and 2-butyne? |
NaNH2 |
The product X in the above reaction is - |
|
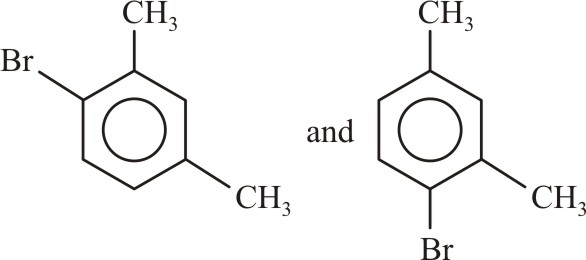
The products formed when meta-xylene is treated with Br2 in the presence of FeBr3 are:-
|
|
A compound that is formed on passing chlorine through propene at 400°C is - |
Allyl chloride |
The reactants used in Friedel-Craft's alkylation are - |
C6H6 + CH3Cl |
The D in the above mentioned reaction is -
|
|
Nitrobenzene on reaction with conc. HNO3/H2SO4 at 80-100°C forms - |
1,3-Dinitrobenzene |
In the following reaction,
|
|
The monochlorinated products (excluding stereo-isomers) obtained from the reaction is:
|
5 |
3-Hexyne can be converted to trans-3-Hexene by the action of: |
Li-Liq. NH3 |
Hydrogen MCQ Questions



Hydrogen Questions and Answers
| Hydrogen Quiz Question | Answer |
|---|---|
Permutit is: |
hydrated sodium aluminium silicate |
Hydrogen is not obtained when zinc reacts with: |
cold water |
The volume strength of 1.5N H2O2 Solution is : |
8.4 |
Density of water is maximum at: |
4°C |
Atomic hydrogen is obtained by: |
passing silent electric discharge through hydrogen at low pressure |
In the preparation of hydrogenated oil the chemical reaction involved is called as- |
Hydrogenation |
The most abundant isotope of hydrogen is- |
Protium |
Hydrogen is evolved by the action of cold dilute HNO3 on: |
Mg or Mn |
A molten ionic hydride on electrolysis gives: |
H2 is liberated at anode |
In solid hydrogen, the intermolecular bonding is: |
van der Waals' |
Organic Chemistry Basic Principles Techniques MCQ Questions



Organic Chemistry Basic Principles Techniques Questions and Answers
| Organic Chemistry Basic Principles Techniques Quiz Question | Answer |
|---|---|
Which is incorrect about enantiomorphs? |
They have same configuration |
How many geometrical isomers are possible of the following? CH3-CH=CH-CH=CH-CH3 |
2 |
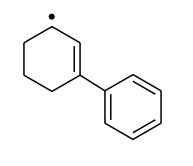
Most stable radical is |
|
Which of the following is the most stable carbonium ion? |
|
Which of the following will have minimum C-Cl bond length? |
Ph-Cl |
The maximum number of alkyl groups in C8H18 is: |
6 |
Compounds which rotate plane polarised light clockwise direction are known as: |
Dextrorotatory |
The decreasing order of stability is-
|
D>C>B>A |
Glucose and fructose are- |
Functional isomers |
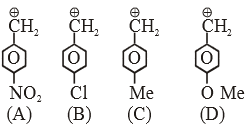
Which among the following is most basic? |
|
Pblock Elements Xi MCQ Questions



Pblock Elements Xi Questions and Answers
| Pblock Elements Xi Quiz Question | Answer |
|---|---|
The incorrect statement among the following is - |
GeCl2 is more stable than GeCl4 |
Silicon carbide is used as: |
Abrasive |
An incorrect statement among the following regarding Al2Cl6 is- |
Six Al—Cl bonds are of same length and two of different length |
Heating an aqueous solution of aluminium chloride to dryness will give: |
Al2O3 |
Carbon dioxide is a gas but silica is a solid because: tetrahedral structure |
carbon dioxide is composed of discrete covalent CO2 molecules whereas silica has continuous |
When a mixture of diborane and ammonia is heated, the final product is |
B3N3H6 |
The greatest percentage of CO is in: |
water gas |
Which of the following shows bond in silicone? |
—|Si—O—|Si—O—|Si— |
Mica is chemically: |
potassium alumino silicate having sheet structure |
Borazine is obtained by reaction of: |
NH3+B2H6 in 2 : 1 ratio |
Pblock Elements Xii MCQ Questions



Pblock Elements Xii Questions and Answers
| Pblock Elements Xii Quiz Question | Answer |
|---|---|
Only iodine forms hepta-fluoride IF7, but chlorine and bromine give Penta-fluorides. The reason for this is : |
that the larger iodine atom can accommodate more number of smaller fluorine atom around it |
Chloride of an element A gave a neutral solution in water. In the periodic table the element A belongs to |
First group |
The correct relationship between the pH of isomolar solutions of sodium oxide(pH1), sodium sulphide (pH2), sodium selenide (pH3) and sodium telluride (pH4) is - |
pH1 > pH2 > pH3 > pH4 |
NO2 can be produced by heating - |
AgNO3 |
The formation of PH+4 is difficult as compared to NH+4 because |
Lone pair of phosphorous resides in almost pure s - orbital. |
Concentrated nitric acid reacts with iodine to give: |
HIO3 |
Partial Hydrolysis of one mole of peroxodi-sulphuric acid produces |
One mole of sulphuric acid, one mole of peroxomono-sulphuric acid |
Which of the following is the correct statement? |
Boiling point increases down the group in halogens |
Incorrectly matched characteristic is: |
S8: covalent lattice |
N2 cannot be produced by heating - |
NH4Cl+CaO |
Polymers MCQ Questions



Polymers Questions and Answers
| Polymers Quiz Question | Answer |
|---|---|
Natural rubber has - |
All cis-configuration |
Perlon is also known as - |
Nylon-6 |
Acrilan is a hard, wool-like feel and a high melting material. Which of the following represents its structure? |
(-CH2-CH|CN-)n |
The polymer that can be classified as polyster polymer is : |
Terylene |
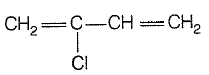
Monomer of neoprene is: |
|
Which one of the following monomers gives the polymer neoprene on polymerization? |
|
Structures of some common polymers are given. The incorrect representation among the following is:
|
Neoprene |
Nylon-6, 10 is a polymer of: |
Hexamethylenediamine and sebacic acid |
Cellulose is a condensation polymer of |
β-glucose |
Toluene di-isocyanate is used to prepare |
polyurethanes |
Redox Reactions MCQ Questions



Redox Reactions Questions and Answers
| Redox Reactions Quiz Question | Answer |
|---|---|
Which of the following is redox reaction ? |
Oxides of nitrogen form nitrogen & oxygen from lightning |
Which of the following is a redox reaction ? |
2Na[Ag(CN)2]+Zn→Na2[Zn(CN)4]+2Ag |
5mL of NHCl, 20 mL of N/2 H2SO4 and 30mL of N/3 HNO3 are mixed together and volume made one litre. The normality of the resulting solution is: |
N/40 |
When a metal is burnt, its mass is increased by 24 percent. The equivalent mass of the metal will be: |
33.3 |
1g of pure calcium carbonate was found to require 50 mL of dilute HCl for complete reactions. The strength of the HCl solution is given by: |
0.4N |
The equivalent mass of H3PO4 in the following reaction is, H3PO4 + Ca(OH)2 →CaHPO4 + 2H2O |
49 |
1.520g of the hydroxide of a metal on ignition gave 0.995 g of oxide. The equivalent mass of metal is: |
9.00 |
0.5 g of fuming H2SO4 (oleum) is diluted with water. This solution is completely neutralised by 26.7 mL of 0.4 N NaOH. The percentage of free SO3 in the sample is: |
20.6% |
One g of a mixture of Na2CO3 and NaHCO3 consumes y equivalent of HCl for complete neutralisation. One g of the mixture is strongly heated, then cooled and the residue treated with HCl. How many equivalent of HCl would be required for complete neutralisation? |
y equivalent |
The chloride of a metal contains 71% chlorine by mass and the vapour density of it is 50. The atomic mass of the metal will be: |
29 |
Sblock Elements MCQ Questions



Sblock Elements Questions and Answers
| Sblock Elements Quiz Question | Answer |
|---|---|
LiAlH4 is obtained by reacting an excess of ...... with an etheral solution of AlCl3. |
LiH |
Manufacture of NaOH is done by: |
Castner - Kellner process |
Sodium does not form Na2+ ,because: |
First ionization potential is small and the difference in first and second ionization potential is very large. |
Lithium is the only alkali metal which is not placed in kerosene but is wrapped in paraffin wax, because: |
it floats to the surface of kerosene because of low density |
Alkali metals are characterised by: |
Good conductors of heat and electricity. |
Excess of dilute sodium hydroxide solution is gradually added with shaking to an aqueous solution of zinc sulphate. What would you observe? |
A white precipitate appears which dissolves to give a colourless solution. |
Which has lowest thermal stability? |
Li2CO3 |
An alkali metal that reacts with nitrogen to form nitride is - |
Li |
Caustic soda is: |
Deliquescent |
The most basic oxide among the following is: |
Na2O |
Solid State MCQ Questions



Solid State Questions and Answers
| Solid State Quiz Question | Answer |
|---|---|
On the basis of unit cell concept a crystal has : |
7 systems |
If NaCl is doped with 10-4 mol % of SrCl2, the concentration of cation vacancies will be (NA = 6.023 x 1023 mol-1) |
6.023 x 1017 mol-1 |
The phenomenon in which polar crystals on heating produce electricity is called:- |
Pyro-electricity. |
Total volume of atoms present in a face centred cubic unit cell of a metal is - (r is atomic radius) |
16/3 πr3 |
Lithium borohydride (LiBH4), crystallizes in an orthorhombic system with 4 molecules per unit cell. The unit cell dimensions are:- a = 6.81 Å , b = 4.43 Å ,c = 7.17 Å. If the molar mass of LiBH4 is 21.76 g mol-1.The density of the crystal is:- |
0.67 g cm-3 |
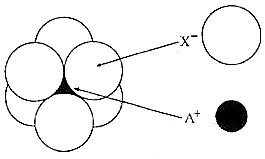
The arrangement of X- ions around A+ ion in solid AX is given in the figure (not drawn to scale).
|
104 pm |
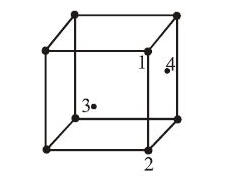
In an f.c.c. unit cell. atoms are numbered as shown below. The atoms not touching each other are (Atom numbered 3 is face centre of front face). |
1&2 |
A solid has a bcc structure. If the distance of closest approach between the two atoms is 1.73Å . The edge length of the cell is: |
200pm |
Which is covalent solid? |
All of these |
What type of crystal defects is indicated in the diagram given below? |
Schottky defect |
Solutions MCQ Questions



Solutions Questions and Answers
| Solutions Quiz Question | Answer |
|---|---|
Which one of the following is incorrect for ideal solution ? |
∆Gmix=0 |
Which gas when passed through dilute blood will impart a cherry red colour to the solution? |
CO |
The vapour density of undecomposed N2O4 is 46. When heated, vapour density decreases to 24.5 due to its dissociation to NO2. The percent dissociation of N2O4 at the final temperature is: |
87 |
At 298 K, 500cm3 H2O dissolved 15.30 cm3 CH4(STP) under a partial pressure of methane of one atm. If Henry's law holds, what pressure is required to cause 0.001 mole methane to dissolve in 300cm3 water ? |
2.33 atm |
The molal depression constant for water=1.85 deg/molal and for benzene is 5.12 deg/molal. If the ratio of the latent heats of fusion of benzene to water is 3:8, calculate the freezing point of benzene. |
5.12°C |
Which of the following colligative properties is associated with the concentration term 'molarity'? |
Osmotic pressure |
The vapour pressure of a dilute aqueous solution of glucose is 750 mm of mercury at 373 K. The mole fraction of solute in the solution is- |
1/76 |
Which one of the folowing pairs of solution can we expect to be isotonic at the same temperature- |
0.1 (M) Ca(NO3)2 and 0.1 (M) Na2SO4 |
The latent heat of vapourisation of water is 540 cal g-1 at 100°C. Kb for water is 1. 0.56 K.mole-1 2. 1.86 K.mole-1
|
3 |
Which of the following aqueous solution has osmotic pressure nearest to that an equimolar solution of K4[Fe(CN)6]? |
Al2(SO4)3 |
States Matter MCQ Questions



States Matter Questions and Answers
| States Matter Quiz Question | Answer |
|---|---|
A gas diffuses four time quickly as oxygen. The molecular weight of gas is: |
2 |
Which among the following mixtures of gases at room temperature does not obey Dalton's law of partial pressure- |
NH3 and HCl |
Themolecular radius for a certain gas = 1.25 A. What is a reasonable estimate of the magnitude of the van der Wails constant, b, for the gas? |
1.97 × 10–2 litre/mole |
At lower temperature, all gases except H2 and He show: |
Negative deviation |
2 gms of hydrogen diffuses from a container in 10 minutes. How many gms of oxygen would diffuse through the same time under similar conditions? |
8 gm |
The mean free path (λ) of a gas sample is given by: |
λ=1/√2πσ2N |
Which is lighter than dry air? |
Moist air |
The ratio a/b (the terms used in van der Waals' equation) has the unit- |
All of the above |
At relatively high pressure, van der Waals' equation reduces to- |
PV = RT + Pb |
The maximum deviation from ideal gas behaviour takes place - |
At low temperature and high pressure |
Structure Atom MCQ Questions



Structure Atom Questions and Answers
| Structure Atom Quiz Question | Answer |
|---|---|
The number of d- electrons retained in Fe2+ : |
6 |
Which of the following sets of quantum numbers is not possible? |
n = 3, l= 3, m = -3, s = 1/2 |
Which of the following sets of quantum numbers represent an impossible arrangement - n l m s |
3 2 -3 (+)1/2 |
How many unpaired electrons are present in Ni2+ cation (atomic number = 28) |
2 |
Number of unpaired electrons in 1s2 2s2 2p3 is - |
3 |
The quantum numbers of four electrons are given below. n l m s (1) Electron 1 3 0 0 -1/2 (2) Electron 2 4 0 0 1/2 (3) Electron 3 3 2 0 1/2 The correct order of decreasing energy of these electrons is - 1. Electron 3 > Electron 1 > Electron 4> Electron 2 |
2. Electron 4> Electron 2 > Electron 3 > Electron 1 |
Which type of radiation is not emitted by the electronic structure of atoms: |
y-rays |
An absorption line of the lowest frequency in the transition of hydrogen spectra is |
n=3 to n=8 |
If uncertainty in the position of an electron is zero the uncertainty in its momentum will be |
infinite |
The maximum number of atomic orbitals associated with a principal quantum number 5 is- |
25 |
Surface Chemistry MCQ Questions



Surface Chemistry Questions and Answers
| Surface Chemistry Quiz Question | Answer |
|---|---|
Flocculation value is expressed in terms of: |
Millimole per litre. |
Compound that acts as inhibitor for knocking in combustion of petrol is : |
Both (1) and (2) |
Which does not show the Tyndall effect? |
Sugar solution |
Among the following surfactant molecules, the surfactant that forms micelles in aqueous solution at the lower CMC at ambient condition is: |
CH3(CH2)15N+(CH3)3Br- |
Butter is a colloid form in which: |
water is dispersed in fat |
The cotterells precipitator is used to : |
all of the above |
Foam is a colloidal solution of: |
Gaseous particles dispersed in a liquid |
A liquid aerosol is a colloidal system of: |
a liquid dispersed in a gas |
A negative catalyst or inhibitor is one: |
which retards the rate of reaction |
Alum helps in purifying water by: |
Aluminium which coagulates the mud particles. |
Thermodynamics MCQ Questions



Thermodynamics Questions and Answers
| Thermodynamics Quiz Question | Answer |
|---|---|
All the naturally occurring processes, i.e., spontaneous proceed spontaneously in a direction that leads to: |
decrease of free energy |
A gaseous system changes from state A(P1, V1, T1) to B(P2,V2,T2), B to C(P3, V3, T3) and finally from C to A. The whole process may be called: |
cyclic process |
Warming ammonium chloride with sodium hydroxide in a test tube is an example of:
|
open system |
Work done in the reversible adiabatic process is given by: |
nR (γ-1)(T2-T1) |
Change in enthalpy for reaction, 2H2O2(l)→ 2H2O(l) + O2(g) If the heat of formation of H2O2(l) and H2O(l) are -188 and -286 kJ/mol respectively is - |
-196 kJ/mol |
One mole of a non-ideal gas undergoes a change of state (2.0 atm, 3.0 L, 95 K)→(4.0 atm, 5.0 L, 245 K) with a change in internal energy, ∆U = 30.0 L atm. The change in enthalpy (∆H) of the process in L atm is: |
44.0 |
At absolute zero, the entropy of a perfect crystal is zero. This is........... of thermodynamics. |
Third law |
At constant pressure and temperature, the direction of any chemical reaction is one where the...... decrease. |
Gibbs energy |
The work done by a mass less piston in causing an expansion ∆V (at constant temperature), when the opposing pressure, P is variable, is given by: |
W= -∫P∆V |
Entropy decreases during: |
crystallization of sucrose from solution |

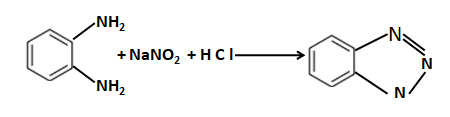
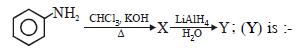
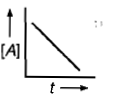
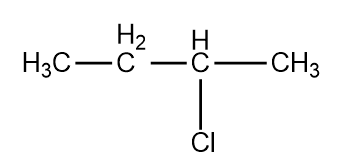 , Cl obtained by chlorination of n-butane, will be
, Cl obtained by chlorination of n-butane, will be 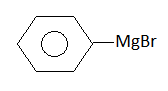 +
+